(+)-gamma-lactamase from microbacterium as well as coding gene and application of (+)-gamma-lactamase
A technology of lactamase encoding and lactamase, which is applied in the field of enzyme engineering, can solve the problems of poor selectivity, restriction, and enzyme instability, and achieve the effects of simple method, low environmental pollution, and good operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
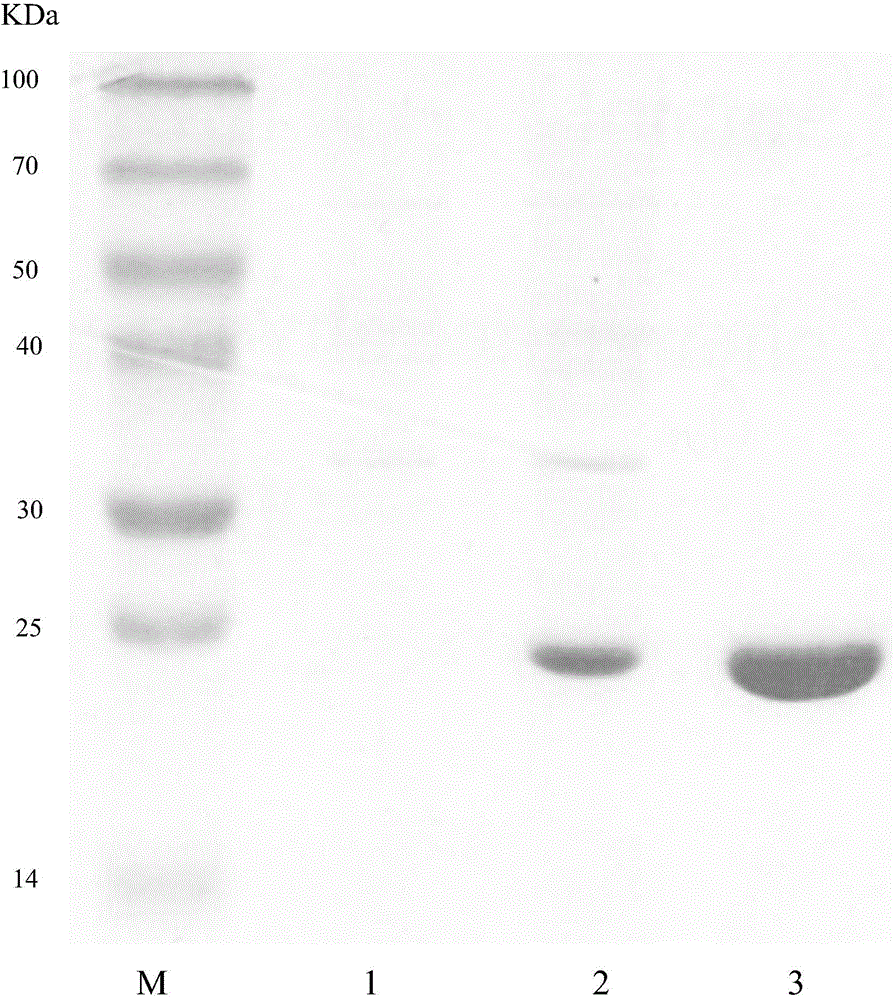
Image
Examples
Embodiment 1
[0044] (1) Acquisition of (+)-γ-lactamase 33H4-5540 gene of the present invention
[0045](1) Primer design
[0046] Primers were designed according to the high-throughput screening 33H4-5540 gene sequence, and the primers were synthesized by Shanghai Sangong.
[0047] 33H4-5540 upstream primer:
[0048] 5'-GGAATTC CATATG GCAGCACCCCGCCGCACCGTCGTCCT-3'
[0049] The underline indicates the Nde I restriction site
[0050] 33H4-5540 downstream primer:
[0051] 5'-CCG CTCGAG TCAGAGAGCGACGTGGTCGTGCGTGGCGAT-3'
[0052] The underline indicates the Xho I restriction site
[0053] (2) PCR amplification of the target gene
[0054] Use the whole genome of Microbacteria as a template for PCR amplification. The PCR reaction system is: 2ul genome, 10ul reaction buffer, 10ul high GC enhancer buffer, 2ul upstream and downstream primers, 2ul DNTP (2.5mM), 0.5ul high-fidelity NEBQ5 polymerase , make up to 50ul with double distilled water. The PCR conditions are: the first step is 98...
Embodiment 2
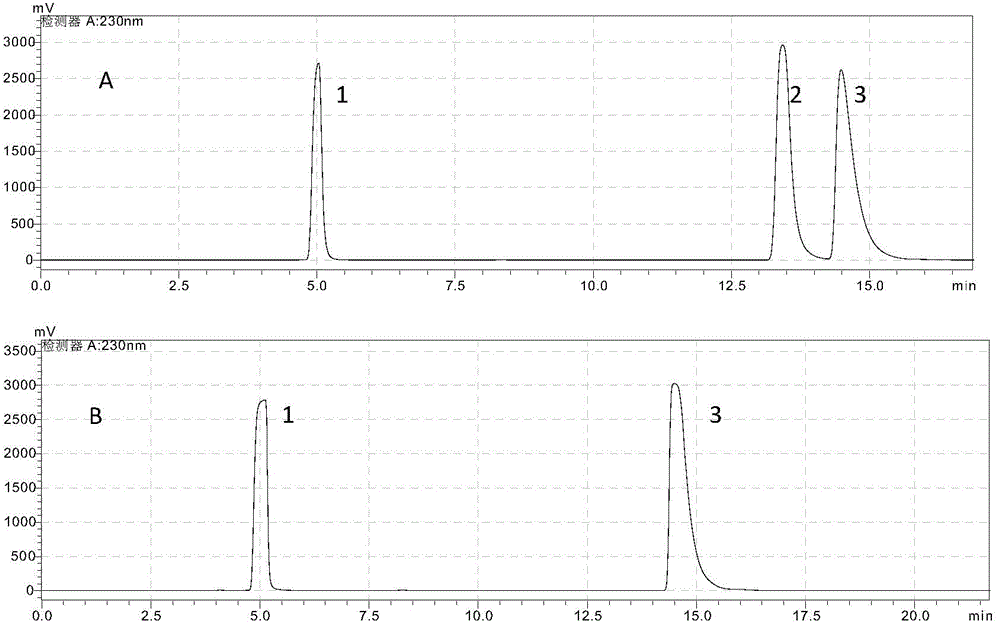
[0066] The quality of the raw materials used in this example is the same as in Example 1, and the preparation method is similar to that of Example 1. The improvement is that in the application of (+)-γ-lactamase to resolve the racemate γ-lactam, an appropriate amount of The recombinant Escherichia coli cells were added to 1 ml of 110 g / l racemic γ-lactam (prepared in phosphate buffer), and reacted at 20° C.-30° C. for 20-30 minutes. After the reaction, the mixed solution was extracted with 1 ml of ethyl acetate to obtain (-)-γ-lactam.
[0067] The (-)-γ-lactam extracted above is subjected to HPLC detection, the optical purity is 99.8%-99.9%, and the yield is greater than 48%.
[0068] The reagents and instruments used in Example 1 and Example 2 are conventional products on the market unless otherwise specified.
[0069] The basic operation techniques of genetic engineering such as PCR, enzyme digestion, connection and transformation adopted in Example 1 and Example 2 are reco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com