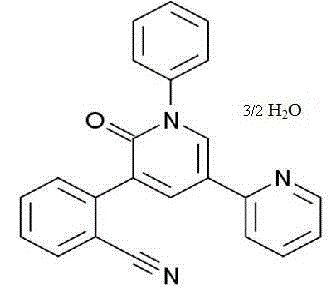
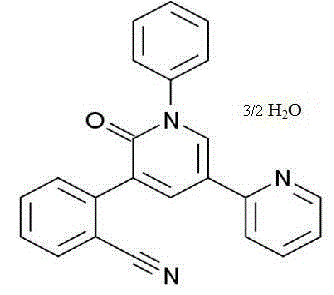
Perampanel sesquihydrate compound
A technology of sesquihydrate and hydrate, which is applied in the field of medicine, can solve the problems of hygroscopic weight gain, difficult purification, high impurity content, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Microparticles or microspheres are prepared by combining the compounds of the present invention with a pharmaceutically acceptable solid or liquid carrier, and optionally with pharmaceutically acceptable adjuvants and vehicles, using standard and conventional techniques. The composition is used for preparing oral preparations and injections. It is given by way of example only and in no way is it intended to limit the scope of the invention in any way.
[0041] Example 2
Embodiment 2
[0043] Prescription: Perampanel sesquihydrate 10.7g, microcrystalline cellulose 12g, pregelatinized starch 16g, sodium starch glycolate 5g, lactose 210g, PEG-4000 25g, magnesium stearate 6g, 30 grams of povidone K30, 33 grams of croscarmellose sodium, appropriate amount of distilled water, made into 10,000 tablets.
[0044] Process:
[0045] Preparation of tablet core: according to the determined prescription, the main ingredient and auxiliary materials are mixed uniformly, granulated, and the granules are ventilated and dried below 40°C, granulated with a 16 mesh sieve, added magnesium stearate and remaining starch, and compressed into tablets to obtain final product .
[0046]Isolation layer coating: add talcum powder to 5% polyvinylpyrrolidone (PVP) absolute ethanol solution, stir evenly, and prepare a 20% suspension as the isolation layer coating solution. Coating is carried out in a fluidized bed, and the process conditions are as follows: spray pressure 0.3 MPa, liquid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com