A method of continuously preparing 1,2-epoxycyclododecane
A technology of epoxy cyclododecane and cyclododecene, which is applied in the direction of organic chemistry, can solve the problems of difficult control of process conditions, unfavorable industrialization, and shortened half-life, and achieve high selectivity of main products, easy industrial scale-up, Realize the effect of industrial amplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~7
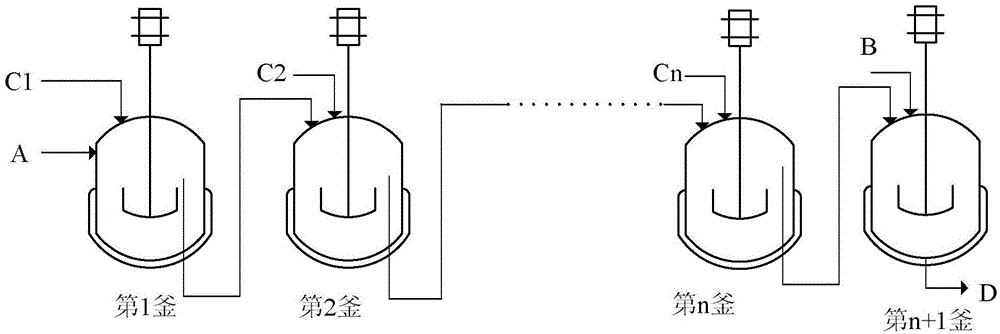
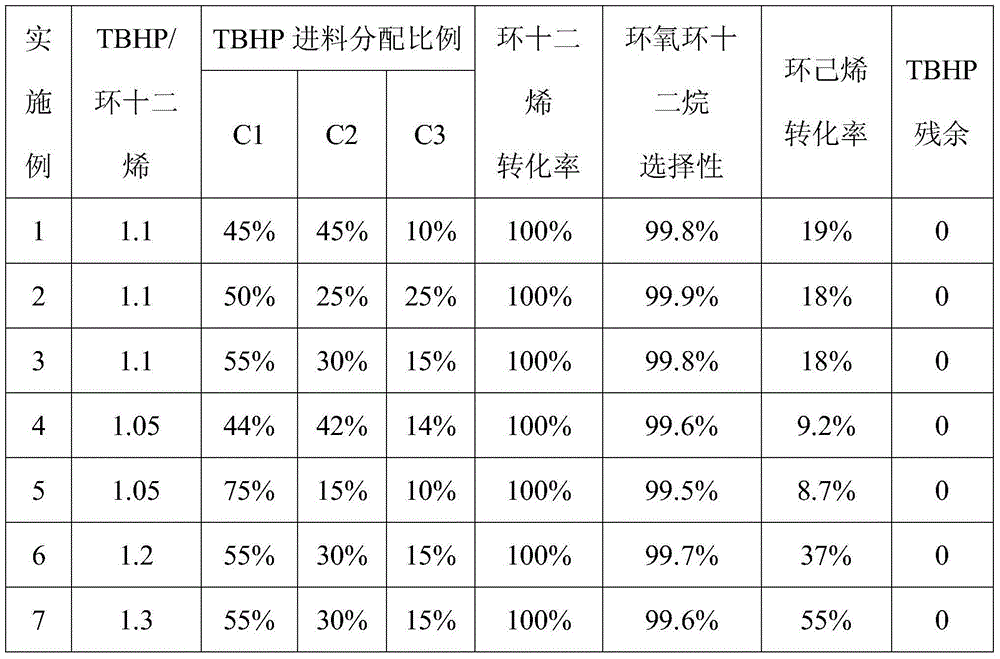
[0051] use figure 1 In the continuous tank reactor shown, where n=3, the reactor is connected in series with four tanks, and the volume of each tank is 1L. Cyclododecene is 0.616kg / h, and the amount of molybdenum acetylacetonate as catalyst is 0.1wt%, based on the weight of cyclododecene. The tert-butanol solution with a TBHP concentration of 50wt% is added according to the total feed amount shown in Table 1 and distributed through the C1-C3 feed inlets, with an average residence time of about 2 hours and a temperature of 110°C. Add cyclohexene 0.152kg / h from B port to the fourth kettle (cyclohexene: cyclododecene=1:2), the residence time is about 0.6h, and the temperature is 90°C. The discharge of discharge port D was analyzed, and the results are shown in Table 1.
[0052] Table 1 Embodiment 1~7 reaction condition and result
[0053]
Embodiment 8
[0055] use figure 1 In the continuous tank reactor shown, where n=10, the reactors are eleven tanks connected in series, and the volume of each tank is 1L. Cyclododecene 0.992kg / h, catalyst ammonium molybdate / ethylene glycol / water complex dosage 0.01wt%, based on the weight of cyclododecene. The total amount of tert-butanol solution with TBHP concentration of 80wt% is 0.807kg / h, according to k 1 ~ k 9 Both are 1 feed, that is, each feed port is fed with 10% of the total amount of TBHP. The 1st to 10th kettles were kept at a reaction temperature of 90°C, and the residence time was 5h. Add 0.490kg / h of cyclohexene to the 11th kettle from port B, and the residence time is about 0.4h. The eleventh kettle was kept at a temperature of 120°C. After the discharge from port D, it is analyzed by gas chromatography. The conversion rate of cyclododecene is 100%, the selectivity of epoxycyclododecane is 99.9%, the conversion rate of cyclohexene is 16%, and there is no remaining TBHP....
Embodiment 9
[0057] use figure 1 In the continuous tank reactor shown, where n=5, the reactor is a series of six tanks, each volume of the first to fifth tanks is 1L, and the volume of the sixth tank is 0.5L. Cyclododecene 0.673kg / h, catalyst molybdenum acetylacetonate dosage 1wt%, calculated by cyclododecene weight. TBHP concentration is 10wt% toluene solution adding total amount is 3.83kg / h, press k 1 ~ k 5Both are 2 feeds, that is, the ratio of each feed port is 51.6% for C1, 25.8% for C2, 12.9% for C3, 6.5% for C4, and 3.2% for C5. The first to fifth kettles were kept at a reaction temperature of 130°C and a residence time of 1h. Add 0.034kg / h of n-hexene to the sixth kettle from port B, and the residence time is about 0.1h. The temperature of the sixth kettle was maintained at 130°C. D mouth is discharged and analyzed by gas chromatography. The conversion rate of cyclododecene is 100%, the selectivity of epoxycyclododecane is 99.8%, the conversion rate of n-hexene is 47%, and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com