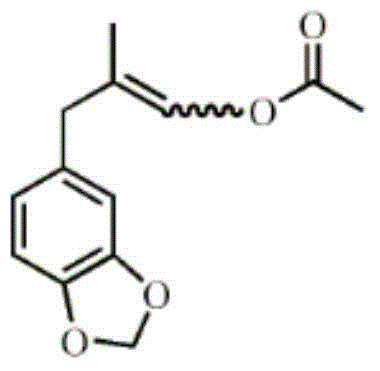
Synthesis process for novel heliotropin monoester
A technology of jasmonal monoester and synthesis process, which is applied in the direction of organic chemistry, can solve the problems of high loss of reactants, poor selectivity, long reaction time, etc., and achieve the effect of reducing synthesis time, reducing operation steps and improving technical solutions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A kind of synthetic technique of new ocean jasmonal monoester, comprises the following steps:
[0032] (1) Esterification reaction: under certain temperature conditions, add acetic anhydride to methacrolein, after the reaction is complete, an esterification intermediate is obtained;
[0033] (2) Alkylation reaction: under certain temperature conditions, piperonyl cyclocyclene is added to the esterification intermediate in step (1), and the reaction is completely separated after alkali washing and water washing to separate the new jasmonal monoester.
[0034] Wherein, the catalyst used in the step (1) esterification reaction and the step (2) alkylation reaction is boron trifluoride diethyl ether, and the catalyst consumption in the step (1) esterification reaction adopts a catalytic equivalent, expressed as methacrolein The amount of the catalyst is 0.001-0.05 in terms of the amount of the substance, and the amount of the catalyst in the alkylation reaction in step (2) i...
Embodiment 2
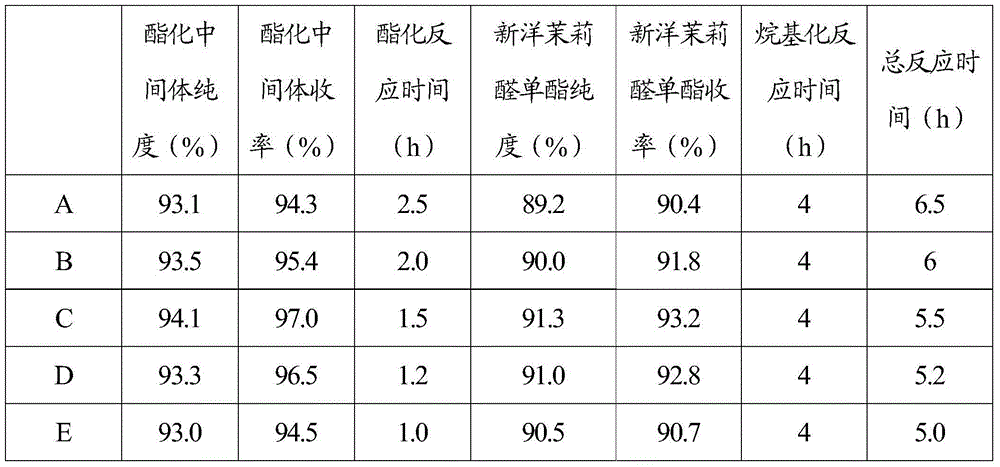
[0043] Adopt the synthetic technique of embodiment one to synthesize new jasmonal monoester, concrete reaction condition is: the temperature of step (1) esterification reaction is-10-5 ℃; Step (2) in the alkylation reaction, piperonyl ring and esterification The molar ratio of the intermediate is 4-6, and the alkylation reaction temperature is 0-5° C. According to the difference in the molar ratio of methacrolein and acetic anhydride in step (1), 5 comparative examples are set and numbered respectively, respectively The molar ratio of methacrolein to acetic anhydride: 0.5-1(1), 1-1.1(2), 1.1-1.25(3), 1.25-1.5(4), 1.5-2(5).
[0044] The resulting product after the reaction is sampled respectively and detected by LC / MS to obtain the purity and yield of the esterification intermediate in step (1) and the purity and yield of step (2) new jasmonal monoester, and record the reaction time, Experimental data as table 2 .
[0045] Table 2 Each product detection data result of embo...
Embodiment 3
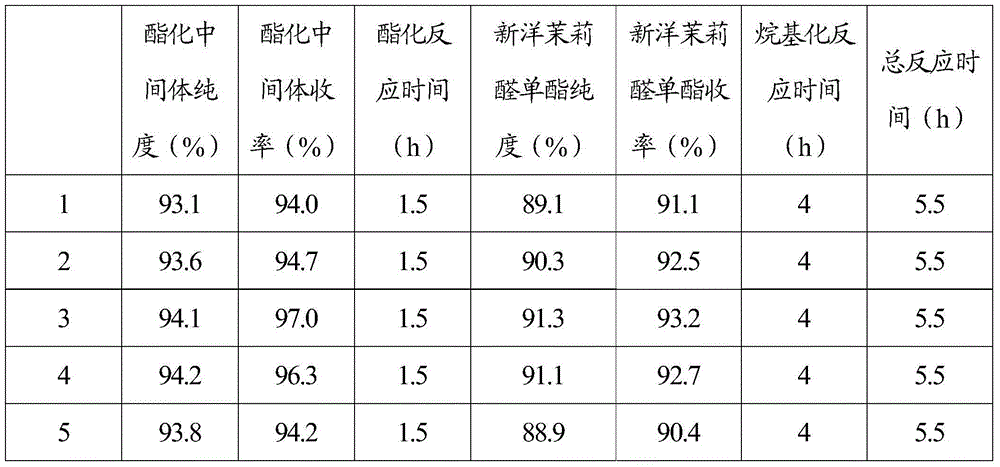
[0049] Adopt the synthesis process of Example 1 to synthesize new jasmonal monoester, the specific reaction conditions are: the temperature of step (1) esterification reaction is -10-5 ℃, the molar ratio of methacrolein and acetic anhydride is 1.1-1.25 ; In the step (2) alkylation reaction, the mol ratio of piperonyl ring and the esterification intermediate is 4-6, according to the difference of the alkylation temperature in the step (2) 4 comparative examples are set and numbered respectively, are respectively alkyl Melting temperature: -10—0°C (Ⅰ), 0—5°C (II), 5—20°C (Ⅲ), 20—40°C (Ⅳ).
[0050] The resulting product after the reaction is sampled respectively and detected by LC / MS to obtain the purity and yield of the esterification intermediate in step (1) and the purity and yield of step (2) new jasmonal monoester, and record the reaction time, Experimental data as table 3 .
[0051] table 3 Each product detection data result of embodiment three
[0052]
[0053] As...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com