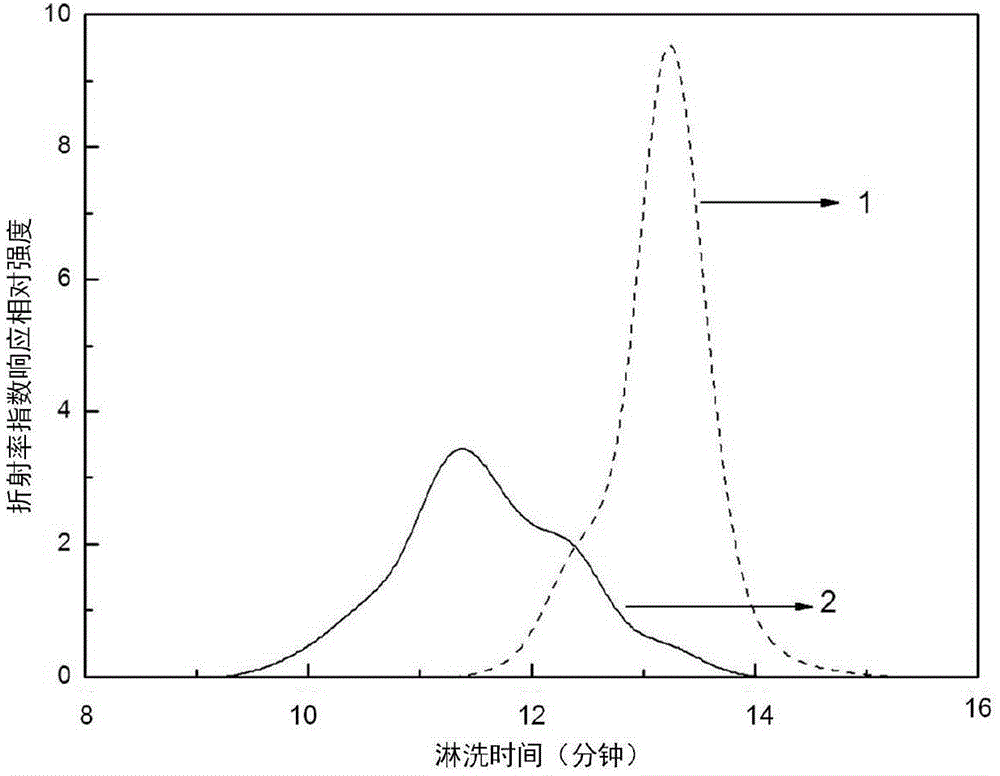
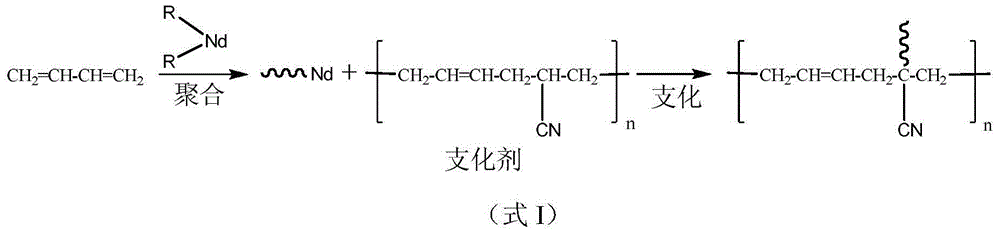
Preparation method of branched polymer
A polymer and branching agent technology, which is applied in the field of preparation of branched polymers, can solve the problem of high price, achieve the effect of strong electron pulling ability, lower solution viscosity, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Under the protection of argon, add 50mL cyclohexane to the dry catalyst preparation bottle, then add 0.03mmol neodymium isooctanoate, 0.75mmol triisobutylaluminum, 0.15mmol diethylaluminum hydride, 0.09mmol diethyl chloride The aluminum was mixed evenly and reacted at room temperature for 1 hour to obtain a rare earth catalyst, which was set aside.
[0039] Under argon protection, add 0.3mol butadiene cyclohexane solution to the dry polymerization reactor, then add the above prepared catalyst solution, the molar ratio of neodymium isooctanoate to butadiene is 1×10 -4 . After polymerization at 50°C for 5 hours, a liquid butadiene-acrylonitrile copolymer was added, wherein the molar ratio of nitrile group to neodymium isooctanoate in the copolymer was 48, and the reaction was continued at 70°C for 0.5h. After the reaction, 1 wt% ethanol solution of 2,6-di-tert-butyl-p-cresol was added to terminate the reaction. After the polymer was washed, it was placed in a vacuum oven...
Embodiment 2
[0045] Under the protection of argon, add 30mL cyclohexane to the dry catalyst preparation bottle, then add 0.04mmol neodymium nonanoate, 0.6mmol triisobutylaluminum, 0.4mmol dibutylaluminum hydride, 0.16mmol diisobutyl chloride The base aluminum was mixed uniformly and reacted at room temperature for 0.5 h to obtain a rare earth catalyst, which was set aside.
[0046] Under the protection of argon, add 0.6mol butadiene and 0.2mol isoprene cyclohexane solution to the dry polymerization reactor, then add the catalyst solution prepared above, the molar ratio of neodymium nonanoate to monomer 5×10 -5 . After 4 hours of polymerization at 55°C, a liquid hydrogenated butadiene-acrylonitrile copolymer was added, wherein the molar ratio of nitrile groups to neodymium nonanoate in the copolymer was 29, and the reaction was continued at 60°C for 0.6h. After the reaction, 1 wt% ethanol solution of 2,6-di-tert-butyl-p-cresol was added to terminate the reaction. After the polymer was was...
Embodiment 3
[0052] Under argon protection, add 95mL hexane to the dry catalyst preparation bottle, then add 0.02mmol neodymium neodecanoate, 0.8mmol diisobutylaluminum hydride, 0.04mmol sesquiethylaluminum chloride and mix well The reaction was carried out for 1 h to obtain a rare earth catalyst, which was set aside.
[0053] Under the protection of argon, add 1mol of butadiene hexane solution to the dry polymerization reactor, then add the catalyst solution prepared above, the molar ratio of neodymium neodecanoate to monomer is 2×10 -5 . After polymerization at 70°C for 5 hours, a liquid butadiene-styrene-acrylonitrile copolymer was added, the molar ratio of nitrile group to neodymium neodecanoate in the copolymer was 8, and the reaction was continued at 80°C for 1 hour. After the reaction, add 1wt% ethanol solution of 2,6-di-tert-butyl-p-methylphenol to terminate the reaction. After the polymer is washed, it is placed in a vacuum oven at 45°C and dried to constant weight. The yield is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com