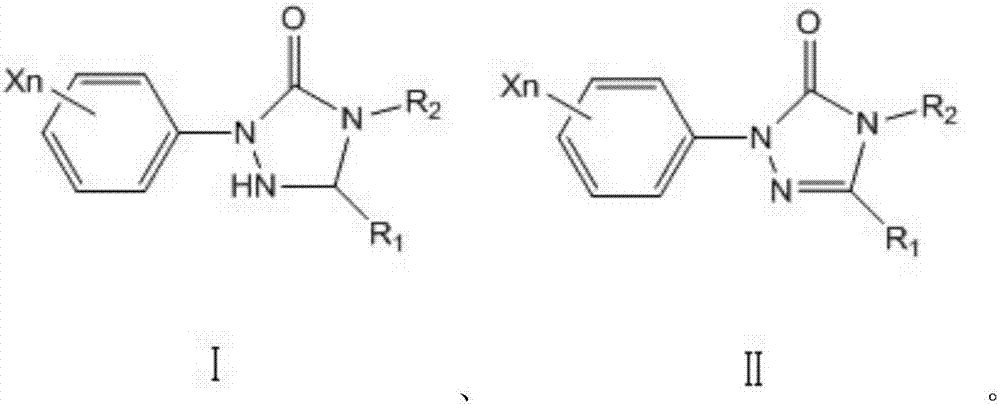
A kind of preparation method of aryl triazolone
A technology for aryl triazolinone and aryl triazolidinone is applied in the field of preparing aryl triazolinone, which can solve problems such as pollution and wastewater treatment difficulties, achieve high purity and yield, and achieve obvious economic and environmental benefits. , the effect of high product content and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
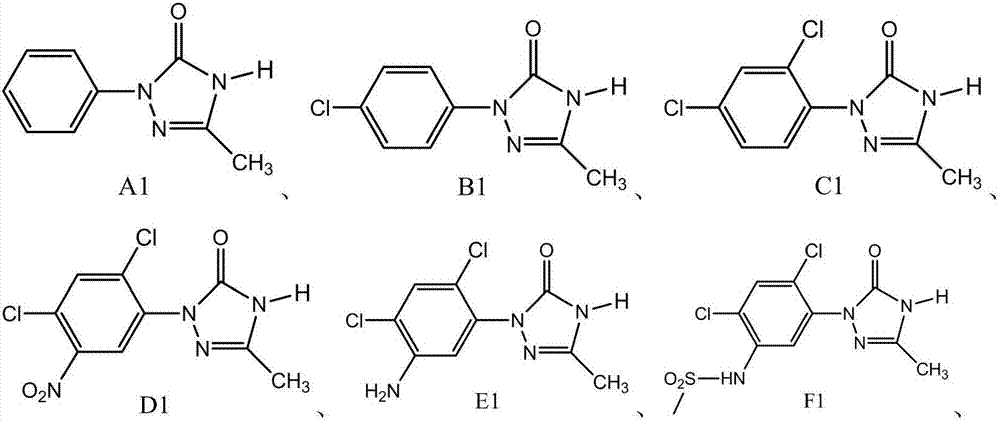
[0033] In a 500ml four-necked flask, add a mixed solution of 250g tert-butanol and water (the mass ratio of tert-butanol and water is 85:15), 50g99.0% of 5-methyl-2-phenyl-1,2,4-tri Oxazolidin-3-one, 1g activated carbon (specific surface area 2000-3000m 2 / g, micropore diameter 0.5~3.0nm), heated to 70 ℃ after fully stirring, slowly added dropwise the hydrogen peroxide 69.2g of 27.5wt%, stirred 60 minutes after dropping, filtered while hot, obtained wet filter cake 2g, will The filtrate was heated to 100°C to remove the solvent, then cooled to 15°C, filtered after crystallization for 1 hour, the filter cake was washed with 5 g of deionized water, and the filter cake was dried to obtain 48.8 g of solid A1, which was 99.0% by gas phase detection. Based on 5-methyl-2-phenyl-1,2,4-triazolidin-3-one, the yield is 98.7%.
Embodiment 2
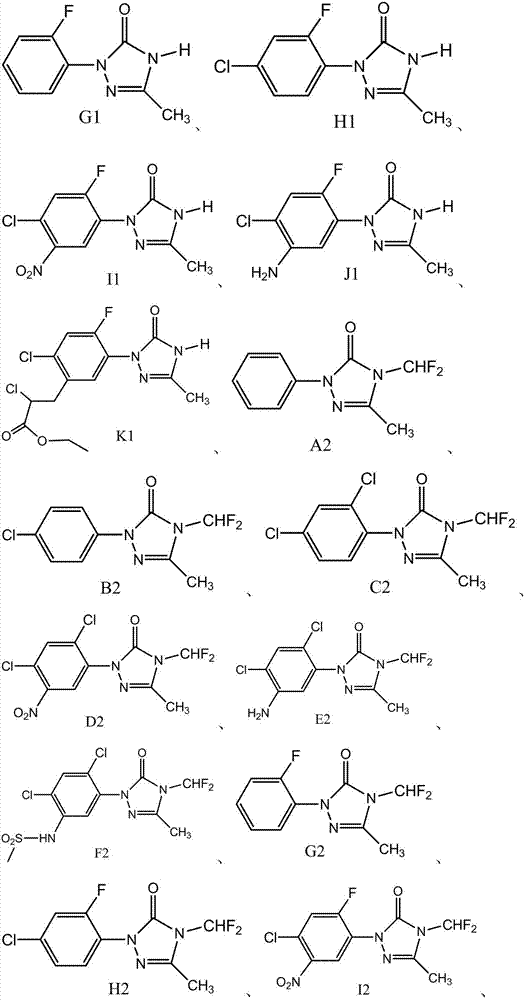
[0035] In the 500ml four-necked flask, add the mixed solution of 250g tert-butanol and water (the mass ratio of tert-butanol and water is 9:1), 50g99.0% 2-(2-fluorophenyl)-5-methyl-1, 2,4-Triazolidin-3-one, 2.5g activated carbon (specific surface area 2000-3000m 2 / g), fully stirred and heated to 50°C, slowly fed 8.1g of pure oxygen, the reaction pressure was 0.3MPa, and the pressure was maintained for 100 minutes after the oxygen was passed through, and then filtered while it was hot to obtain 5g of wet filter cake, and the filtrate was heated to 100 ℃ to remove the solvent, then lower the temperature to 15 ℃, filter after crystallization for 1h, wash the filter cake with 5g deionized water, dry the filter cake to obtain a total of 48.6g of solid G1, the gas phase detection content is 98.8%, and the 2-( Based on 2-fluorophenyl)-5-methyl-1,2,4-triazolidin-3-one, the yield is 98.0%.
Embodiment 3
[0037] In the 500ml four-necked flask, add the mixed solution of 250g tert-butanol and water (the mass ratio of tert-butanol and water is 85:15), 50g99.0% N-(2,4-dichloro-5-(4-(di Fluoromethyl)-3-methyl-5-oxo-1,2,4-triazolidin-1-yl)phenyl)methanesulfonamide, 1.5g activated carbon (specific surface area 1500-2500m 2 / g), fully stirred and then heated to 40°C, slowly fed 5g of gas containing 50% by volume of oxygen, the reaction pressure was 1.0MPa, kept pressure and reacted for 100 minutes after passing through oxygen, then cooled to 60°C and filtered to obtain 3g of wet filter cake , the filtrate was heated to 100°C to remove the solvent, then cooled to 15°C, crystallized for 1 hour and filtered, the filter cake was washed with 5g of deionized water, and the filter cake was dried to obtain a total of 49.7g of solid F2, the content of which was 98.0 by gas phase detection. %, as N-(2,4-dichloro-5-(4-(difluoromethyl)-3-methyl-5-oxo-1,2,4-triazolidin-1-yl)phenyl ) methanesulfona...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com