Preparation method of polyferric sulfate
A technology for polymerizing ferric sulfate and ferrous sulfate, which is applied in the fields of ferric sulfate, flocculation/sedimentation water/sewage treatment, etc., which can solve the problems of product performance failing to meet the requirements of first-class products, increased dosage of hydrogen peroxide, and production costs Increase and other issues, to achieve the effect of promoting conversion rate, reducing dosage, and reducing cost input
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
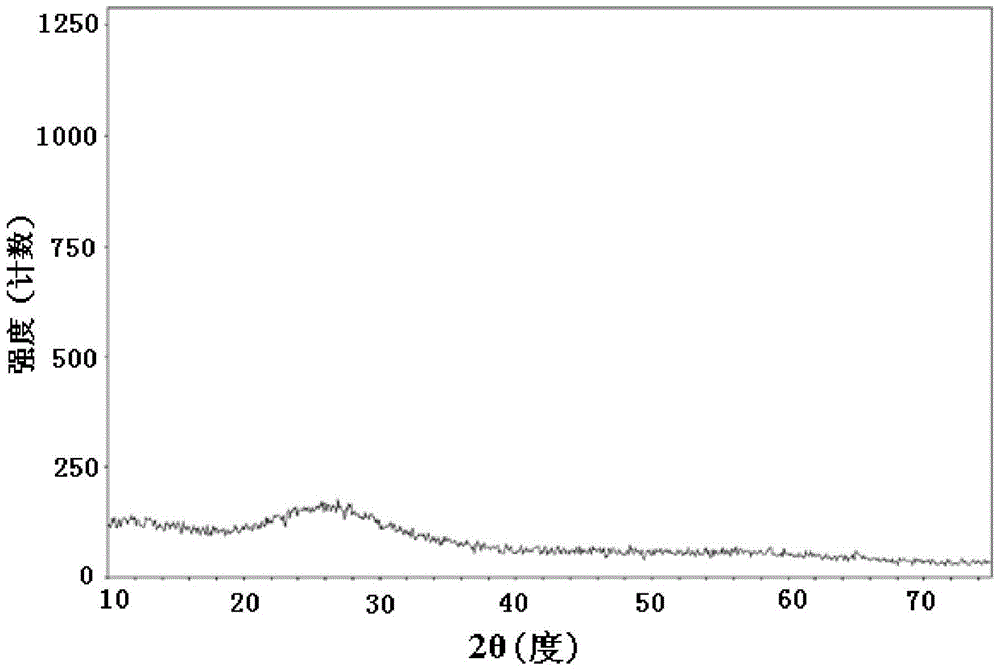
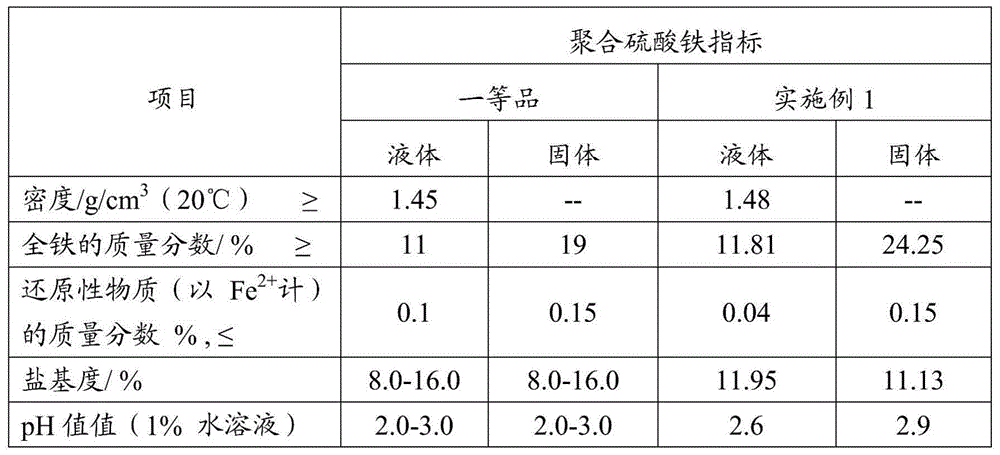
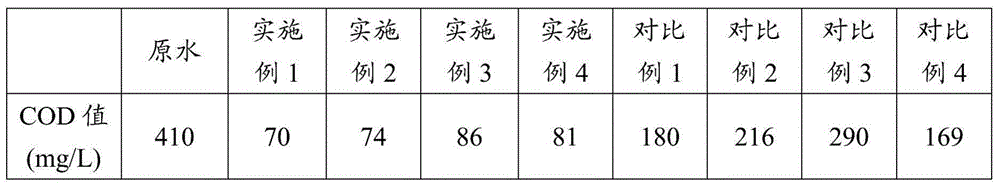
Image
Examples
Embodiment 1
[0035] The preparation method of the polyferric sulfate described in the present embodiment, comprises the steps:
[0036] (1) get the sulfuric acid aqueous solution that sulfuric acid content is 60wt% to 2.75Kg, ferrous sulfate heptahydrate content is that the industrial grade ferrous sulfate heptahydrate solid of 91wt% carries out acidification treatment, and stirring makes it acidify evenly until obtaining pH=1 Mixed solution, the mass concentration of ferrous ions in the mixed solution is 250g / L;
[0037] (2) Under normal temperature and pressure, the mixed solution and the hydrogen peroxide with a hydrogen peroxide content of 25wt% are realized to flow separately through separate flow channels, after the mixed solution and the hydrogen peroxide flow out from the respective flow channels , the two are in contact with each other and react at a height of 4 / 5 of the total height of the reactor from the bottom of the reactor; wherein, the flow rate of the mixed liquid is contr...
Embodiment 2
[0040] The preparation method of the polyferric sulfate described in the present embodiment, comprises the steps:
[0041] (1) getting sulfuric acid content is that the sulfuric acid aqueous solution of 65wt% carries out acidification treatment to the ferrous sulfate solid of 2.75Kg, obtains the mixed solution of pH=0.5, and the mass concentration of ferrous ion is 200g / L in the described mixed solution;
[0042] (2) Under normal temperature and pressure, the mixed solution and the hydrogen peroxide with a hydrogen peroxide content of 50 wt% are realized to flow separately through separate flow channels, after the mixed solution and the hydrogen peroxide flow out from the respective flow channels , the two are in contact with each other and react at a height of 7 / 10 of the total height of the reactor from the bottom of the reactor; wherein, the flow rate of the mixed liquid is controlled to be 70mL / min, and the flow rate of the hydrogen peroxide is 10mL / min min, so that the mo...
Embodiment 3
[0045] The preparation method of the polyferric sulfate described in the present embodiment, comprises the steps:
[0046] (1) getting the sulfuric acid aqueous solution that sulfuric acid content is 70wt% carries out acidification treatment to the waste ferrous sulfate solid 2.75Kg that produces in the titanium dioxide production process, obtains the mixed solution of pH=1, the quality of ferrous ion in the described mixed solution Concentration is 220g / L, contains the ferrous sulfate of 90wt% in the described waste ferrous sulfate solid;
[0047] (2) At normal temperature and pressure, the mixed solution and the hydrogen peroxide with a hydrogen peroxide content of 37.5wt% are realized to flow separately through separate flow channels, when the mixed solution and the hydrogen peroxide flow out from the respective flow channels Afterwards, the height of the two from the bottom of the reactor is 2 / 3 of the total height of the reactor and reacts; wherein, the flow rate of the m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com