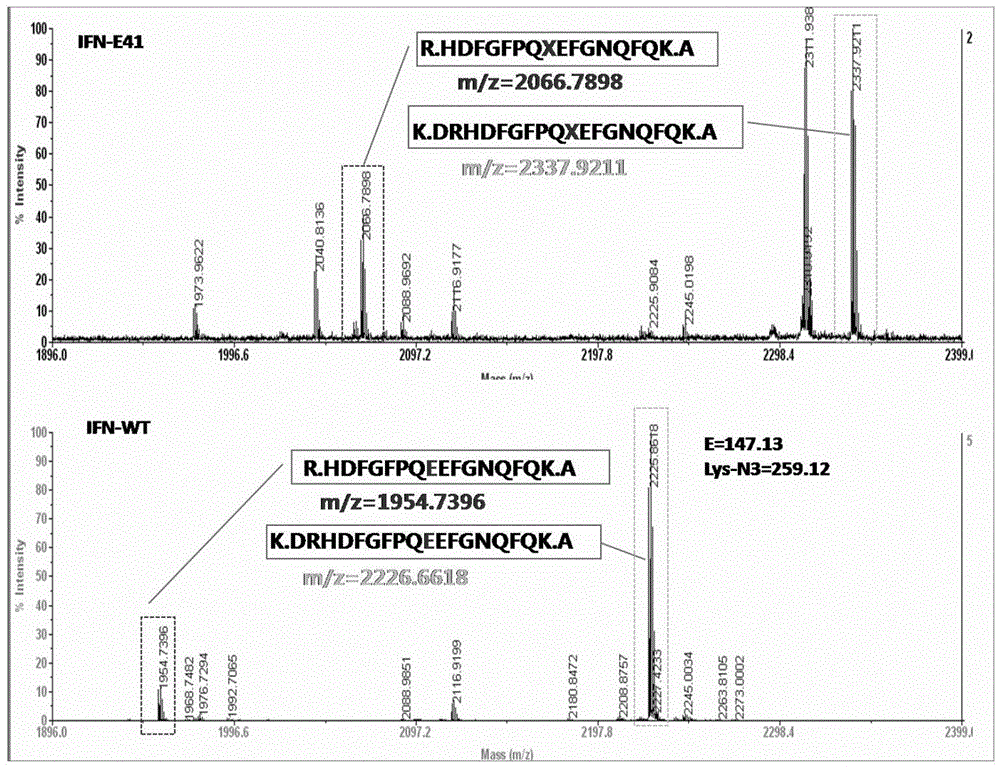
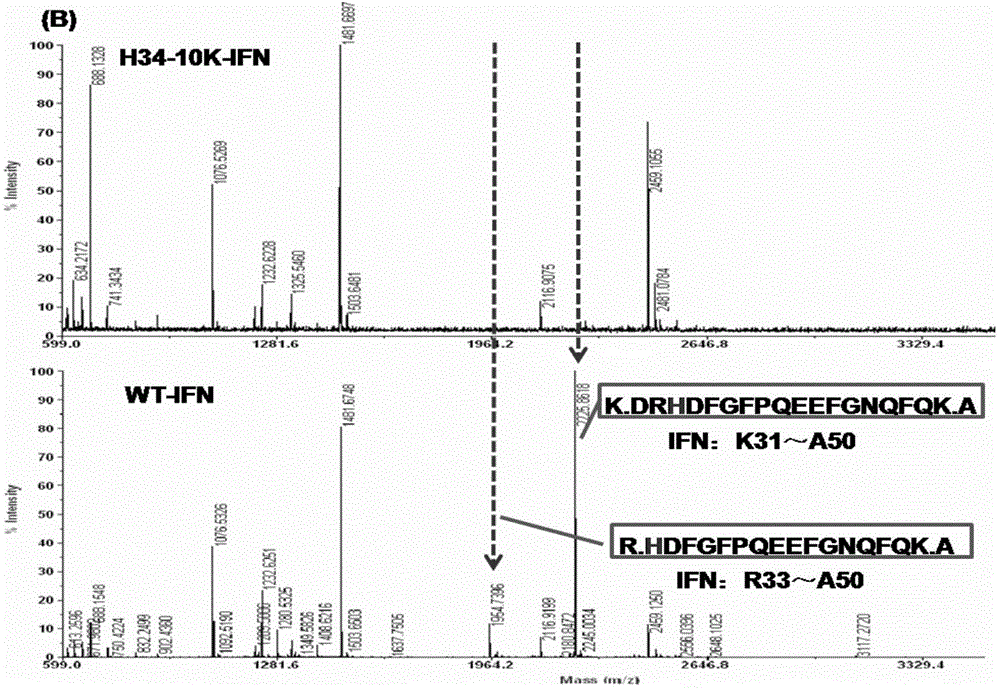
Improved PEGylated recombinant human interferon alpha 2 b
An interferon, specific site technology, applied in the direction of interferon, cytokine/lymphokine/interferon, plant gene improvement, etc., can solve the problems of high post-processing, difficult quality control, unfavorable large-scale production and preparation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1: the construction of the gene vector comprising the human interferon of site-directed mutation
[0088] (1) Acquisition of natural human interferon plasmid helper plasmid
[0089] The interferon gene (SEQ ID NO: 2) was obtained through whole gene synthesis. Then connect it to the pET-21a(+) expression vector with 6*His tag to obtain the expression plasmid of natural interferon (pET21a(+)-IFN(WT); Center (Strain Preservation Address: No. 1 Beichen West Road, Chaoyang District, Beijing, Institute of Microbiology, Chinese Academy of Sciences, the preservation date is April 8, 2013, and the preservation number is CGMCC No: 7432. The classification is named Escherichia coli (Escherichia coli)) Escherichia coli pSUPAR-YAV-tRNA / PylRS containing plasmid pSUPAR-YAV-tRNA / PylRS was obtained from the plasmid pSUPAR-YAV-tRNA / PylRS (hereinafter referred to as the helper plasmid). The tRNA and tRNA synthetase that specifically recognize the unnatural amino acid Lys-az...
Embodiment 2
[0098] Example 2: Expression, purification, PEGylation and identification of interferon with site-directed mutation
[0099] In the present invention, constructing pSUPAR-YAV-tRNA / PylRS plasmid and expressing and pyrrolysyl-tRNA synthetase After the plasmid co-expression, in the host bacteria, in principle, using this protein translation system can make the unnatural amino acid Lys-azido incorporated into the protein, thereby causing site-directed mutation of interferon.
[0100] The inventors experimented with the incorporation possibilities of Lys-azido and identified site-directed PEGylation.
[0101] 1: Lys-azido incorporation expression and purification of mutant interferon
[0102] (1) Culture the expression strain obtained in step 4 of Example 1 in 2*YT medium containing 34ug / ml chloramphenicol and 100ug / ml ampicillin at 37°C for 12-16 hours, and then undergo secondary amplification When the OD value of the bacterial solution reaches 0.6-1.0, add Lys-azido to a f...
Embodiment 3
[0113] Embodiment 3: The interferon mutant that is suitable for modifying obtains
[0114] Amino acid changes in the interferon sequence will affect the properties of interferon. By evaluating the impact of single-point replacement of unnatural amino acids on the in vitro activity of interferon, we will preliminarily identify interferon suitable for unnatural amino acid insertion and subsequent modification. Mutants were screened and identified.
[0115] A. Optical surface plasmon resonance experiment (SPR):
[0116] Interferon α2b exerts biological effects by binding to two receptor units on the cell surface, which we call IFNAR1 and IFNAR2. Among them, IFNAR2 is the main binding unit, which binds to α-type interferon with nanomolar high affinity.
[0117] The optical surface plasmon resonance experiment (SPR) anchors a protein on the surface of the sensor chip, and then flows the sample to be tested over the chip surface. If there are molecules in the sample that can inter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com