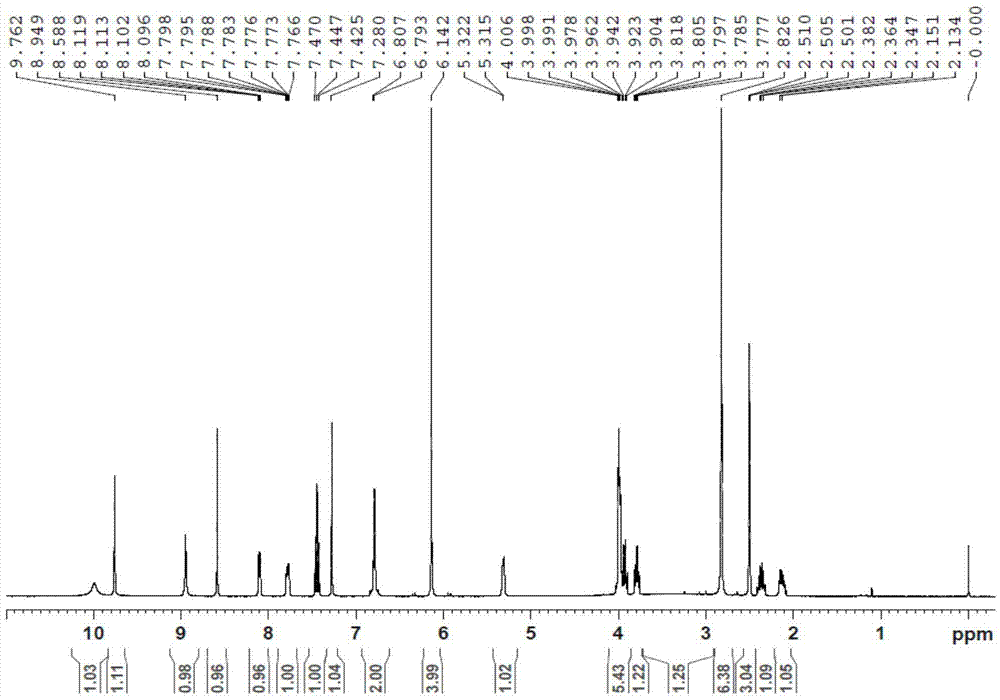
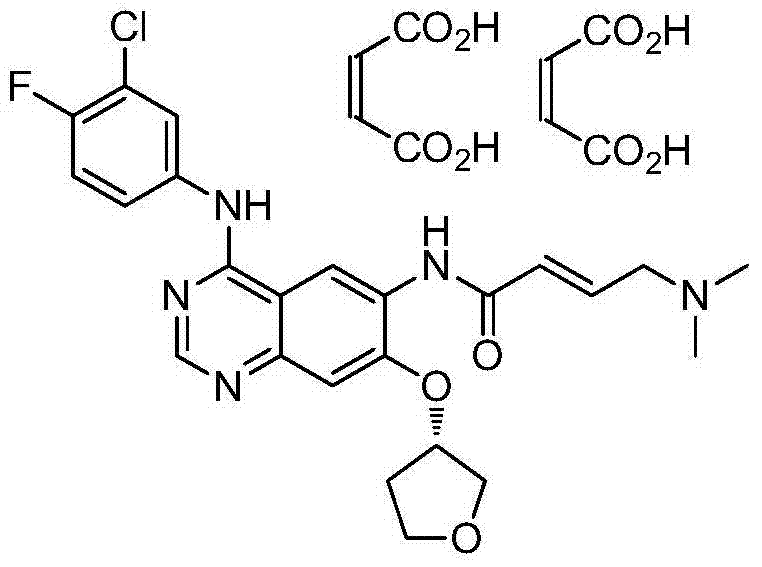
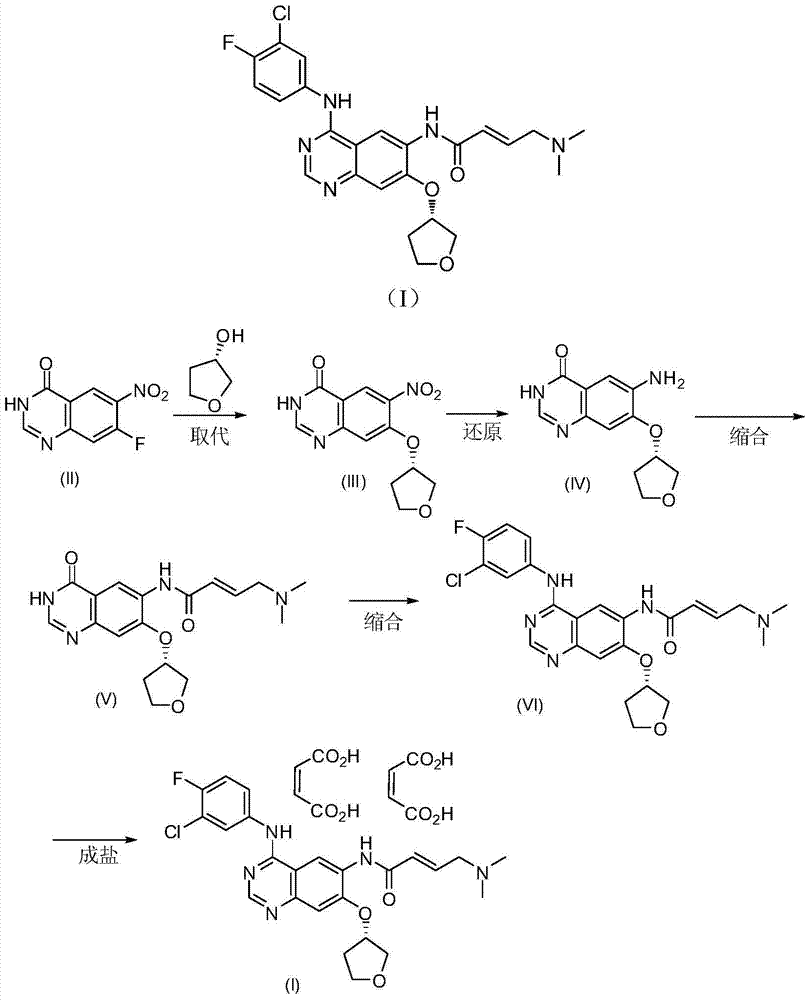
Preparation method of afatinib dimaleate
A technology of afatinib and hexafluorophosphate, which is applied in the fields of organic chemistry and medicinal chemistry, can solve the problems of difficult process control and many steps, and achieves the effects of convenient preparation, short route and reduced production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] At low temperature, add 27.0 g of potassium tert-butoxide into a 500 ml single-necked bottle, stir 100 ml of DMF until dissolved, add 5.5 g of (S)-3-hydroxy-tetrahydrofuran, and keep stirring for one hour. 10 g of compound (II) was added. Keep warm and stir for 2 hours. Add 300ml of water, adjust pH=6-7 with 1.3M hydrochloric acid, stir overnight, filter with suction, and dry to obtain 13g.
Embodiment 2
[0030] Add 4.1g of compound (III) to 66ml of ethanol, 33ml of water and 10ml of acetic acid, heat the oil bath to reflux, add 3.9g of iron powder, and the reaction solution releases gas. It was detected by TLC that the starting material disappeared. Diatomaceous earth filter aid. Prepare a mixed solvent of dichloromethane:methanol=9:1, stir and wash the reaction solution 3 times with 300ml. The combined organic phases were separated, dried, filtered, and concentrated to obtain 3.3 g.
Embodiment 3
[0032] Add 4.135g of CDI and 30ml of THF to a 500ml single-necked bottle in sequence, and stir at 40°C until it dissolves. Add 5 g of diethylphosphonoacetic acid and wash with 7.5 ml of THF. The reaction solution was exothermic, and after stirring for 30 minutes, 5 g of compound (IV) was added. The reaction solution was refluxed, and the TLC reaction was completed. Cool to room temperature, add 300ml of MTBE, stir, filter with suction, and dry under vacuum at room temperature to obtain 9g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com