Hindered amine light stabilizer 3346 preparation method
A technology of light stabilizers and hindered amines, applied in the field of compound preparation, can solve the problems of high energy consumption, post-processing of organic solvents and environmental problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
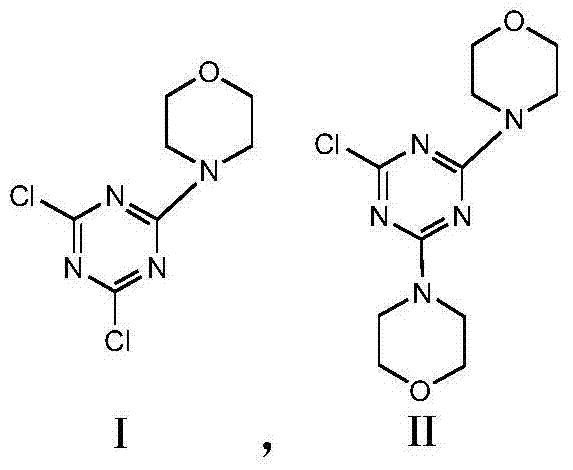
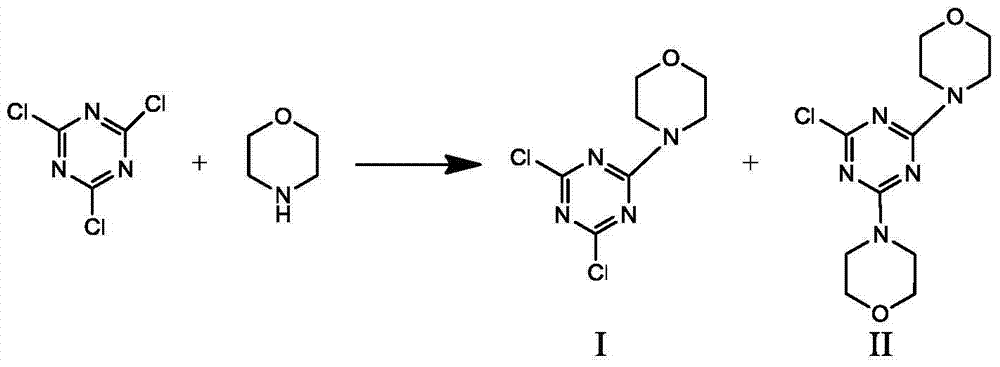
[0032]Add 50.71g (0.275mol) of cyanuric chloride and 200mL of pure water to a 500mL four-neck flask, cool down in an ice bath to below 10°C, add dropwise 30.49g (0.35mol) of morpholine under stirring, maintain 0-10°C, drop Adding time is 1.5h; after dropping, add 10% sodium hydroxide dropwise, containing 14.8g (0.37mol) of sodium hydroxide, keep at 0-10°C, drop for 1h, keep at 0-10°C for 0.5h after dropping ;Heat up to 20°C, add dropwise 10% sodium hydroxide aqueous solution, containing 2.8g (0.07mol) of sodium hydroxide, add dropwise for 0.5h, keep at 20°C for 1h after dropping; heat up to 45°C for 1h, control pH =8~9 for 0.5h. Filter, wash with water to pH = 7, and dry at 80°C to obtain 67.12 g of white powder with a yield of 98.01%. HPLC analysis yields nI:nII = 8:3.
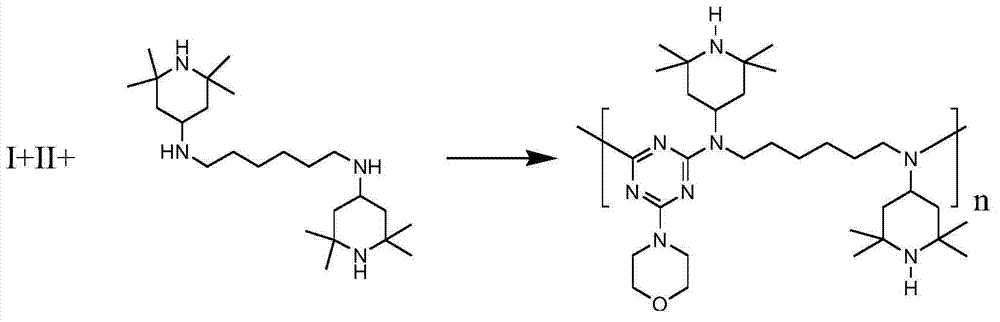
[0033] Add 68.43g of the product obtained by the above method in a 1L autoclave, 90.78g (0.23mol) of hexamethylenediamine piperidine, 400mL of dimethylbenzene, seal, nitrogen replacement under stirring to re...
Embodiment 2
[0035] Add 50.71g (0.275mol) of cyanuric chloride and 200mL of pure water to a 500mL four-neck flask, cool down in an ice bath to below 10°C, add dropwise 25.16g (0.29mol) of morpholine under stirring, maintain 0-10°C, drop Adding time is 1.5h; after dropping, add 10% sodium hydroxide dropwise, containing 11.6g (0.29mol) of sodium hydroxide, keep at 0-10°C, drop for 1h, keep at 0-10°C for 0.5h after dropping ;Heat up to 20°C, add dropwise 10% sodium hydroxide aqueous solution, containing 2.32g (0.058mol) of sodium hydroxide, add dropwise for 0.5h, keep at 20°C for 1h after dropping; heat up to 45°C for 1h, control pH =8~9 for 0.5h. Filter, wash with water to pH = 7, and dry at 80°C to obtain 64.36 g of white powder with a yield of 98.5%. HPLC analysis shows nI:nII = 8:0.5.
[0036] Add 50.58g of the product obtained by the above method into a 1L autoclave, 78.94g (0.2mol) of hexamethylenediamine piperidine, 400mL of xylene, remove the air in the autoclave by nitrogen replacem...
Embodiment 3
[0038] Add 50.71g (0.275mol) of cyanuric chloride and 200mL of pure water to a 500mL four-neck flask, cool down in an ice bath to below 10°C, add dropwise 35.94g (0.41mol) of morpholine under stirring, maintain 0-10°C, drop Adding time is 1.5h; after dropping, add 10% sodium hydroxide dropwise, containing 16.4g (0.41mol) of sodium hydroxide, keep at 0-10°C, drop for 1h, keep at 0-10°C for 0.5h after dropping ;Heat up to 20°C, add dropwise 10% sodium hydroxide aqueous solution, containing 3.28g (0.082mol) of sodium hydroxide, add dropwise for 0.5h, keep at 20°C for 1h after dropping; heat up to 45°C for 1h, control pH =8~9 for 0.5h. Filter, wash with water to pH = 7, and dry at 80°C to obtain 67.12g of white powder with a yield of 98.01%. HPLC analysis converted nI:nII = 2:1.
[0039] Add 75.58g of the product obtained by the above-mentioned method in a 1L autoclave, 94.72g (0.24mol) of hexamethylenediamine piperidine, 400mL of xylene, seal, remove the air in the autoclave by ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com