Beta-glucosaccharase mutant, recombinant expression plasmid thereof and transformed engineering strain
A technology of glucosidase and mutant, applied in the direction of glycosylase, recombinant DNA technology, introduction of foreign genetic material using vectors, etc., can solve the problems of reducing conformational flexibility, enzyme inactivation, organic solvent intolerance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] The implementation methods in the following examples are conventional methods unless otherwise specified.
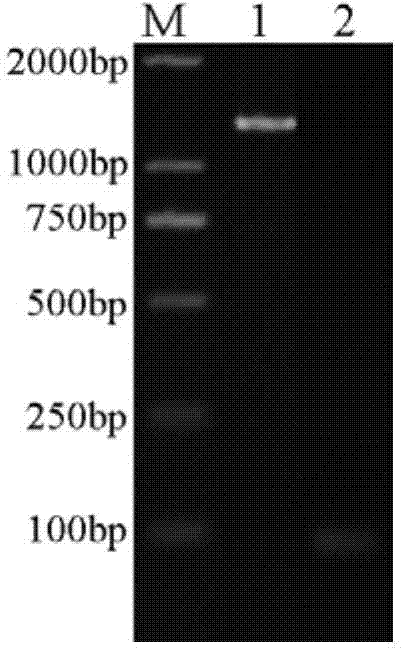
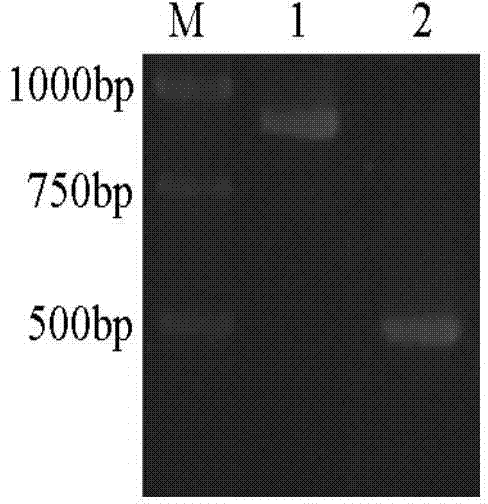
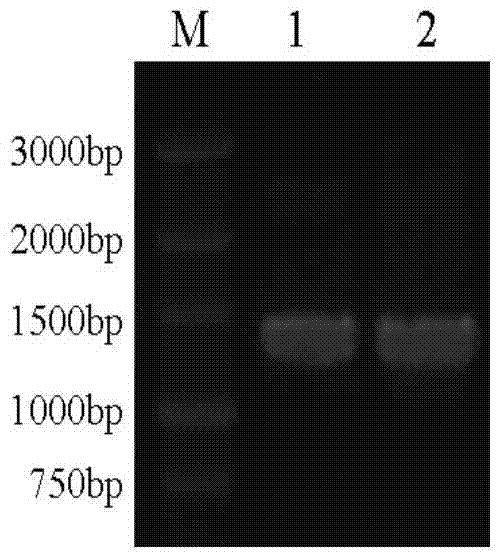
[0029] (1), the construction of the expression bacterial strain that contains β-glucosidase mutant gene of the present invention
[0030] 1. Selection of β-glucosidase gene mutation sites
[0031] Based on sequence alignment, Bgl1A is most similar to β-glucosidase BglB (PDB code: 2O9R) from Paenibacillus polymyxa, with an amino acid sequence identity of 43%. Using the structure of BglB as a template, using the Swiss-Model (http: / / swissmodel.expasy.org / ; Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Research .2009, 37, D387-392.) Modeling the structure of β-glucosidase (Bgl1A) of marine uncultured microbial origin.
[0032] According to the modeled structural information, it is determined that the site-directed mutations are alanine A at position 24 and phenylalanine F at position 297, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com