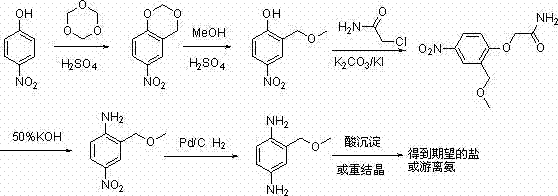
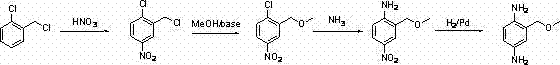
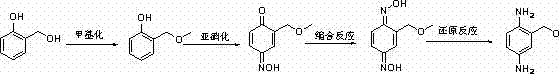
Method for preparing 2-( methoxyl-methyl) phenyl-1,4-diamine
A technology of methoxymethyl and phenyl, which is applied in the field of preparation of 1,4-diphenylamine, can solve the problems of being unsuitable for industrial production, unsuitable for industrial production, expensive reducing agent, etc., and achieve simplified operation and convenient operation , the effect of less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Preparation of o-nitrobenzyl methyl ether:
[0067] At room temperature, add 275g ethyl acetate, 45.9g 0.3mol o-nitrobenzyl alcohol, 38.2g 0.36mol sodium carbonate to a 1000ml four-neck flask, stir for 10min, and slowly add 40.5g 0.45mol carbonic acid dropwise at 20-25°C Dimethyl ester, the dropping time is 1.0h, keep warm at 50~60°C for 0.5h~1.0h after dropping, HPLC central control, stop the reaction when the raw material is less than 1.0%, drop to room temperature, add 100ml of water, continue to stir for 20min , standing still for liquid separation, extracting the aqueous phase with 100ml ethyl acetate, standing to separate layers, combining the organic phases, and distilling under reduced pressure to recover ethyl acetate to obtain a yellow solid, which was blown and dried overnight at 30°C to obtain o-nitrobenzyl Methyl ether: 47.4g, yield: 94.6%, HPLC: 98.5%.
[0068] Preparation of o-aminobenzyl methyl ether:
[0069] At room temperature, add 140.0g of 0.833mo...
Embodiment 2
[0077] Preparation of o-nitrobenzyl methyl ether:
[0078] At room temperature, add 280g of acetonitrile, 45.9g of 0.3mol o-nitrobenzyl alcohol, 38.2g of 0.36mol sodium carbonate into a 1000ml four-necked flask, stir for 10min, and slowly add 40.5g of 0.45mol dimethyl carbonate dropwise at 20-25°C Ester, the dropping time is 1.0h, keep warm at 55~65°C for 0.5h~1.0h after dropping, HPLC central control, stop the reaction when the raw material is less than 1.0%, drop to room temperature, add 100ml of water, continue to stir for 20min, static Separate the liquid, extract the aqueous phase with 100ml ethyl acetate, let stand to separate the layers, combine the organic phases, and distill under reduced pressure to recover the ethyl acetate to obtain a yellow solid, which is blown and dried overnight at 30°C to obtain o-nitrobenzyl methyl ether : 48.3g, yield: 96.4%, HPLC: 98.7%.
[0079] Preparation of o-aminobenzyl methyl ether:
[0080]At room temperature, add 140.0g of 0.833mo...
Embodiment 3
[0088] Preparation of o-nitrobenzyl methyl ether:
[0089] At room temperature, add 280g of acetonitrile, 45.9g of 0.3mol o-nitrobenzyl alcohol, 38.2g of 0.36mol sodium carbonate into a 1000ml four-neck flask, stir for 10min, and slowly add 56.7g of 0.45mol dimethyl sulfate dropwise at 15-25°C Ester, the dropping time is 1.0h, keep warm at 50~60°C for 0.5h~1.0h after dropping, HPLC central control, stop the reaction when the raw material is less than 1.0%, drop to room temperature, add 100ml of water, continue stirring for 20min, static Separate the liquid, extract the aqueous phase with 100ml ethyl acetate, let stand to separate the layers, combine the organic phases, and distill under reduced pressure to recover the ethyl acetate to obtain a yellow solid, which is blown and dried overnight at 30°C to obtain o-nitrobenzyl methyl ether : 47.9g, yield: 95.6%, HPLC: 98.0%.
[0090] Preparation of o-aminobenzyl methyl ether:
[0091] At room temperature, add 140.0g of 0.833mol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com