Anti-grass carp hemorrhagic disease virus engineering protein tat-vp7-tat and its preparation method and application
A technology of artificial preparation and protein, applied in the direction of antiviral agents, biochemical equipment and methods, virus antigen components, etc., can solve the problem that the molecular mechanism is not fully studied, and achieve prevention of grass carp hemorrhagic disease, rapid immune response, and reduced dosage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of fusion gene TAT-VP7-TAT comprises the following steps:
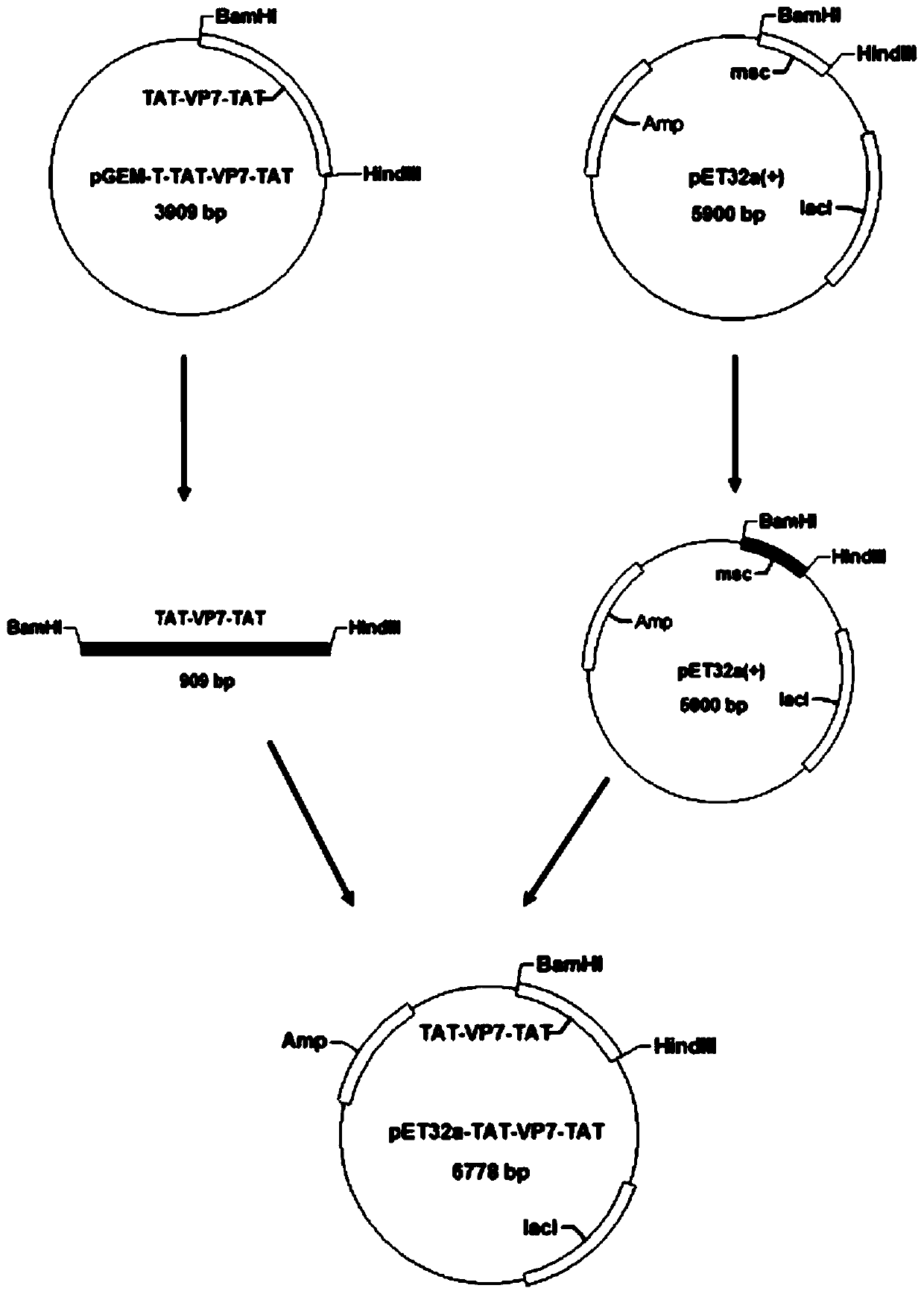
[0044] The amino acid sequence of the mature GCRV VP7 protein was obtained from the gene bank of the National Center for Biotechnology (NCBI), and the sequence of the VP7 gene was converted into a nucleotide sequence containing E. The gene encoded by TAT and the foreign protein gene can be fused and expressed. The 5' and 3' ends of the optimized VP7 gene sequence are respectively connected with the nucleotide sequence of TAT, named: TAT-VP7-TAT, the sequence It is the nucleotide sequence shown in SEQ ID NO:1.
[0045] The TAT-VP7-TAT gene was artificially synthesized and connected to the pGEM-T vector, that is, the plasmid pGEM-T-TAT-VP7-TAT was synthesized.
[0046] The plasmid pGEM-T-TAT-VP7-TAT was transformed into Escherichia coli E. coli DH5α (purchased from Novagen and kept in our laboratory) to obtain strain E.coli DH5α (pGEM-T-TAT-VP7-TAT), For the value increase and preservation of ge...
Embodiment 2
[0056] Construction of expression plasmids.
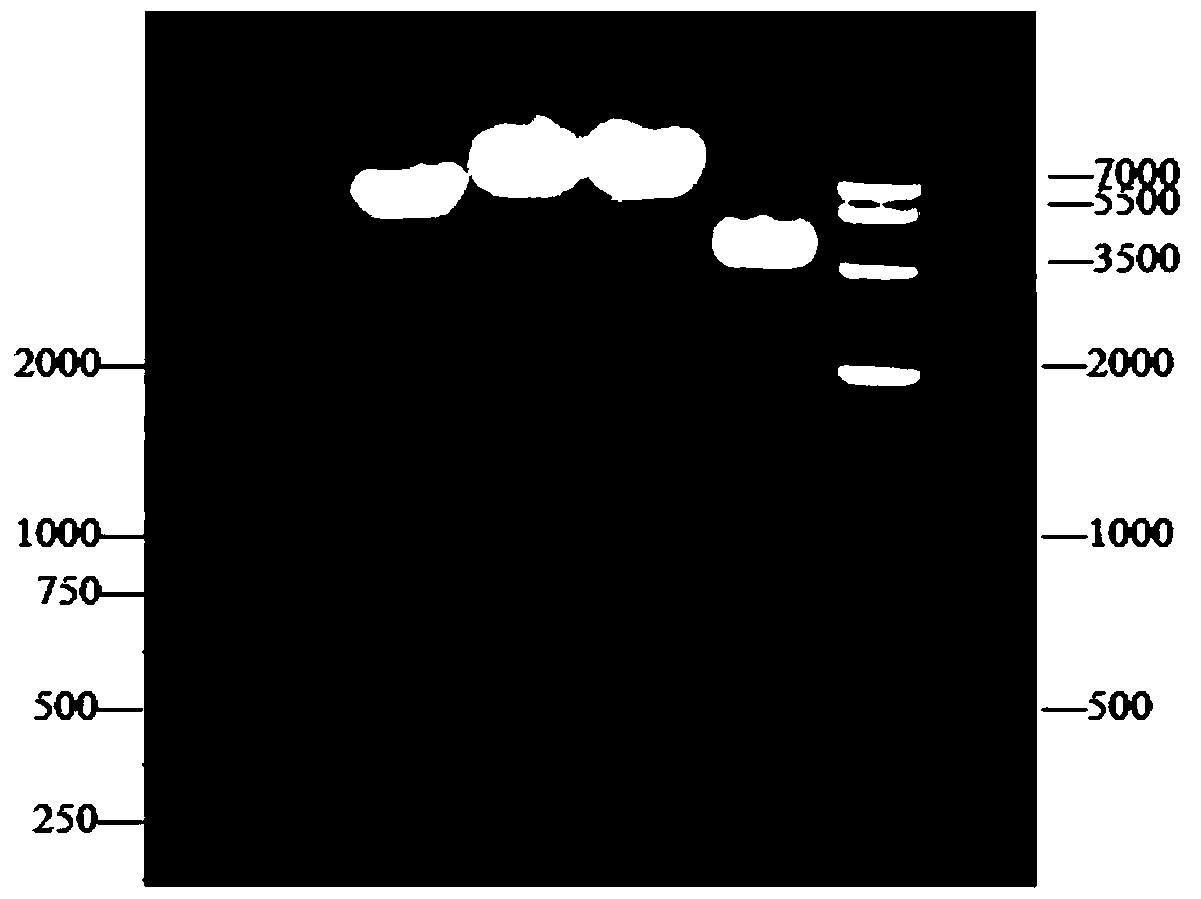
[0057] In Example 1, the plasmid pGEM-T-TAT-VP7-TAT was digested by BamHI and HindIII, electrophoresed on agarose gel, and the band to be recovered was cut out quickly under ultraviolet light, and gelatinized with Kangwei Century agarose. Gel DNA recovery kit for purification, put a single target DNA band into a clean Eppendorf tube, and weigh it. Add three times the volume of sol solution PG to the gel block (the weight of the gel is 0.1g, its volume can be regarded as 100uL, and so on). Water bath at 60°C for 10 minutes, during which time the Eppendorf tube was gently turned up and down every 2 minutes to ensure that the gel was fully dissolved. Add 250 μl of Buffer PS to the adsorption column, let it stand for 2 minutes, then centrifuge at 12000 rpm for 2 minutes, and pour off the liquid in the collection tube. Take 750ul of the obtained solution and add it to an adsorption column (the adsorption column is placed in a collecti...
Embodiment 3
[0071] Construction of pET32a-TAT-VP7-TAT engineering bacteria.
[0072] Take 1 ul plasmid pET32a-TAT-VP7-TAT to transform Escherichia coli BL21(DE3) competent cells. Positive transformants were screened out by PCR identification, and the obtained positive clone was the recombinant genetically engineered strain Escherichia coli BL21pET32a-TAT-VP7-TAT capable of expressing TAT-VP7-TAT.
[0073] The strain was sent to the China Type Culture Collection Center for preservation on March 13, 2015, with a classification name: Escherichia coli BL21(DE3) / pET-32a-TAT-VP7-TAT, and a preservation number: CCTCC NO : M2015111, Address: Wuhan University, Wuhan, China.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com