Compounds and application thereof in preparation of anti-parasitosis drugs
A technology of compound and ketone compound, applied in the field of application in the preparation of antiparasitic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
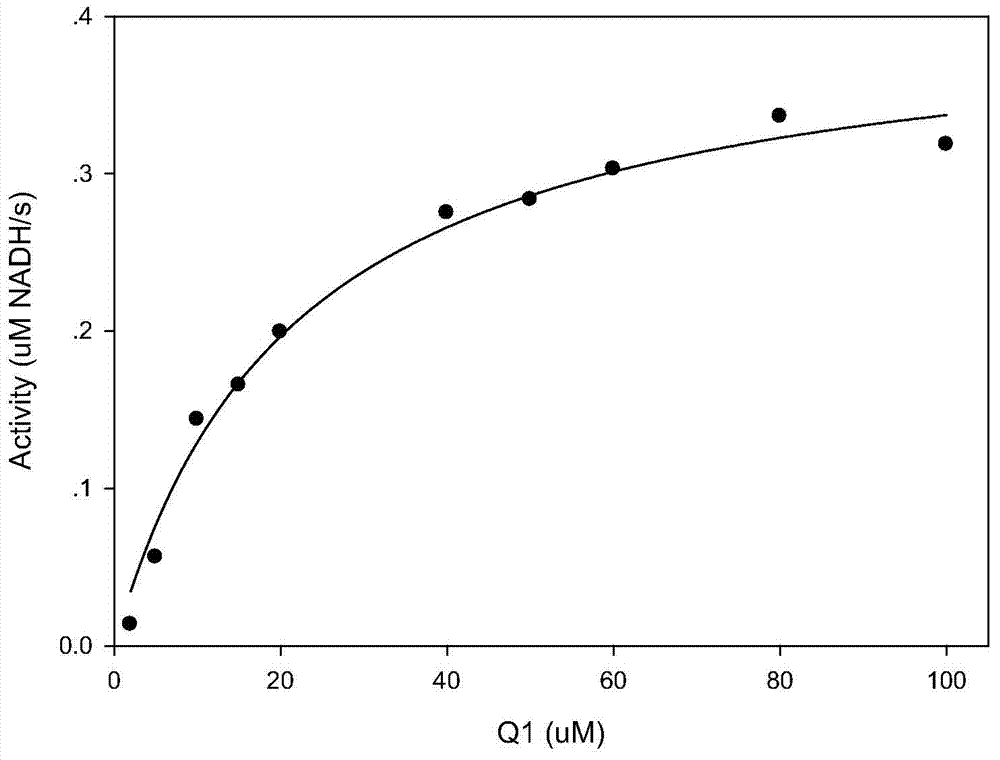
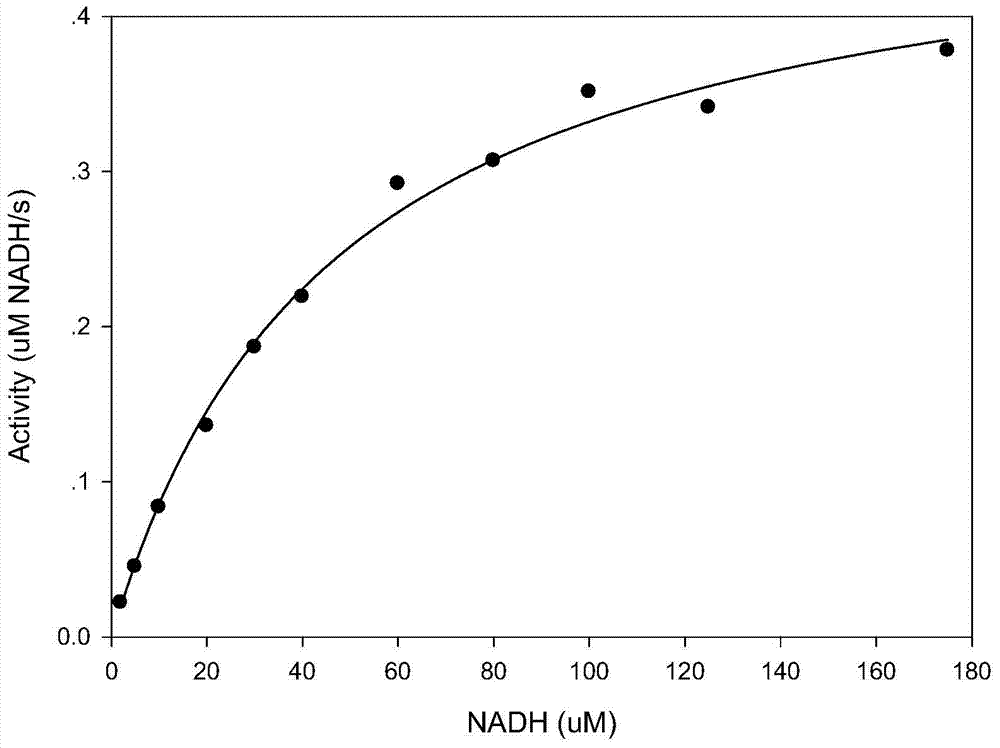
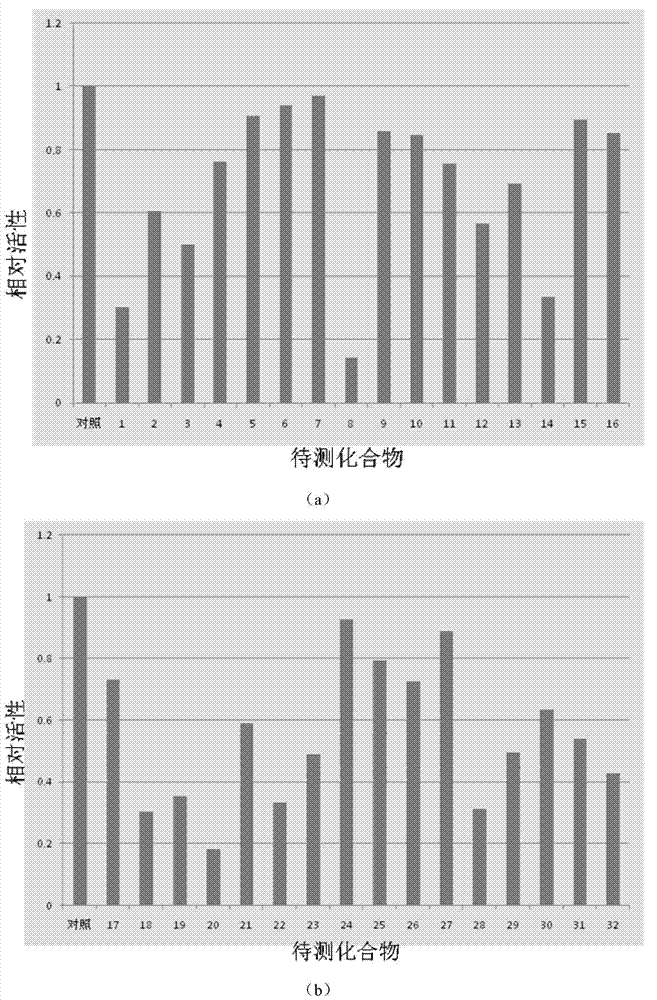
Image
Examples
Embodiment 1
[0142] Example 1. Preparation of compound 1
[0143] The reaction equation is as follows:
[0144]
[0145] Under argon protection, weigh 1.5g of p-bromomethylacetophenone, 2g of p-trifluoromethoxyphenylboronic acid, 32mg of palladium acetate, 74mg of triphenylphosphine and 3g of potassium phosphate into a 100ml round-bottom flask, add 20ml Toluene was reacted at 80°C for 8 hours under argon protection, the solvent was spin-dried under reduced pressure, the residue was dissolved in 20 ml of dichloromethane, washed twice with water, dried over anhydrous sodium sulfate, the solvent was spin-dried under reduced pressure, and the residue was used Petroleum ether:ethyl acetate=30:1 (volume ratio) was passed through a silica gel column to obtain 1.7 g of intermediate A1 (intermediate A1 was a light yellow liquid) with a yield of 81%.
[0146] Take 1g of ethyl o-chlorobenzoate in a 100ml round-bottomed flask, add 20ml of dry tetrahydrofuran to dissolve; weigh 200mg of sodium hydrid...
Embodiment 2
[0150] Example 2, the preparation of compound 2
[0151] The reaction equation is as follows:
[0152]
[0153] Take 1.2g of methyl 2,4-dichlorobenzoate in a 100ml round-bottomed flask, add 20ml of dry tetrahydrofuran to dissolve; weigh 200mg of sodium hydride and add it to the above solution, and stir at 50°C. Take 1 g of intermediate A1 and dissolve it in 5 ml of dry tetrahydrofuran solution, and slowly add the tetrahydrofuran solution of intermediate A1 dropwise to the above solution stirred at 50 °C; then increase the temperature to 80 °C, reflux for about 10h to The reaction ends. The solvent was spin-dried under reduced pressure, the residue was dissolved in dichloromethane, washed twice with water, dried with anhydrous sodium sulfate, the solvent was spin-dried under reduced pressure, and the residue was washed with petroleum ether:ethyl acetate=20:1 (volume ratio) Silica gel column to obtain 842 mg of intermediate A3 (intermediate A3 is a light yellow liquid) in a...
Embodiment 3
[0158] Example 3, the preparation of compound 3
[0159] The reaction equation is as follows:
[0160]
[0161] Take 1.3g of 2,6-dichlorobenzoyl chloride in a 100ml round-bottomed flask, add 20ml of dry tetrahydrofuran to dissolve; weigh 200mg of sodium hydride and add it to the above solution, and stir at 50°C. Take 1 g of intermediate A1 and dissolve it in 5 ml of dry tetrahydrofuran solution, and slowly add the tetrahydrofuran solution of intermediate A1 dropwise to the above solution stirred at 50 °C; then increase the temperature to 80 °C, reflux for about 10h to The reaction ends. The solvent was spin-dried under reduced pressure, the residue was dissolved in dichloromethane, washed twice with water, dried with anhydrous sodium sulfate, the solvent was spin-dried under reduced pressure, and the residue was washed with petroleum ether:ethyl acetate=20:1 (volume ratio) Silica gel column to obtain 541 mg of intermediate A4 (intermediate A4 is a light yellow liquid) in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Absorption coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com