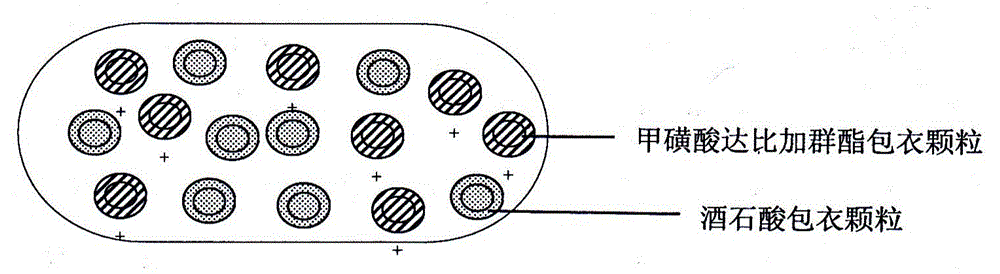
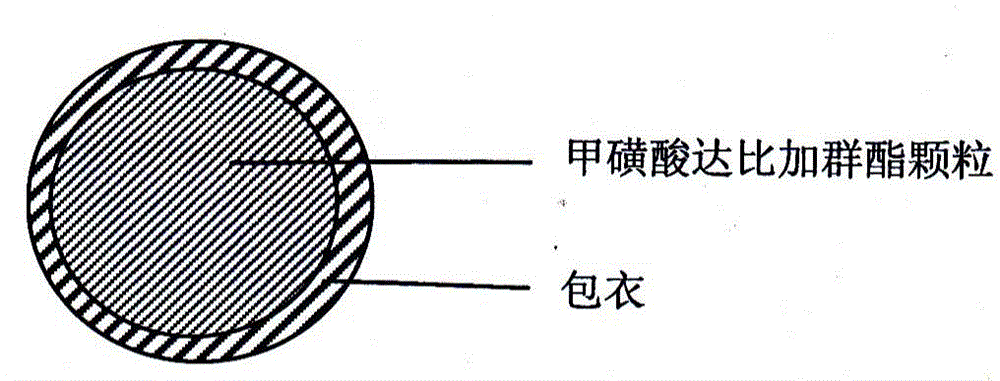
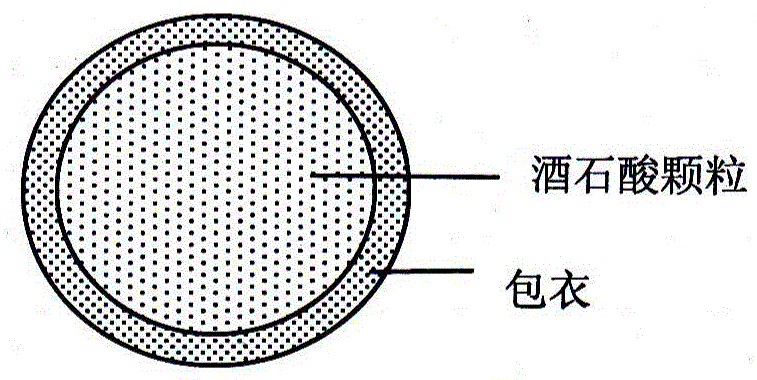
A dabigatran etexilate mesylate capsule pharmaceutical composition and a preparing method thereof
A technology of dabigatran etexilate mesylate and composition, which is applied in the field of pharmaceutical preparations, can solve the problems of long time consumption, poor patient compliance, complicated process, etc., and achieve the effects of good dissolution effect, improved compliance, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
[0028] The preparation of embodiment 1~3 dabigatran etexilate mesylate capsules
[0029] Prescription: a prescription for preparing 1000 dabigatran etexilate mesylate capsules
[0030]
[0031] Preparation Process:
[0032] The first part: the preparation of dabigatran etexilate mesylate coated granules
[0033] (1) Granulation: get the hypromellose of prescription quantity 40%, add appropriate amount of isopropanol and be mixed with 2% solution, standby as binder; Add binder in the dabigatran etexilate mesylate , granulation; dry the wet granules and granulate;
[0034] (2) Coating: get the remaining hypromellose, add appropriate amount of isopropanol to be mixed with 2% solution, add the talcum powder of 2.2% of the total amount of the first part of the prescription, stir well, set aside; The sized granules are placed in a fluidized bed and coated to obtain the finished product.
[0035] Part II: Preparation of Tartaric Acid Coated Granules
[0036] (1) Granulation: ...
Embodiment 4~6
[0039] The preparation of embodiment 4~6 dabigatran etexilate mesylate capsules
[0040] Prescription: a prescription for preparing 1000 dabigatran etexilate mesylate capsules
[0041]
[0042] Preparation Process:
[0043] The first part: the preparation of dabigatran etexilate mesylate coated granules
[0044] (1) Granulation: get 40% polyvinylpyrrolidone of prescription quantity, add appropriate amount of isopropanol and be mixed with 3% solution, standby as binder; Add binder in dabigatran etexilate mesylate, prepare Granules; dry the wet granules and then granulate;
[0045] (2) Coating: take the remaining polyvinylpyrrolidone, add appropriate amount of isopropanol to prepare a 3% solution, add 2.2% talcum powder in the first part of the prescription, stir well, and set aside; The final granules are placed in a fluidized bed and coated to obtain the finished product.
[0046] Part II: Preparation of Tartaric Acid Coated Granules
[0047] (1) Granulation: Take 10% ...
Embodiment 7
[0050] The preparation of embodiment 7 dabigatran etexilate mesylate capsules
[0051] Prescription: a prescription for preparing 1000 dabigatran etexilate mesylate capsules
[0052]
[0053] Preparation Process:
[0054] The first part: the preparation of dabigatran etexilate mesylate coated granules
[0055] (1) Granulation: get 2.8g hydroxypropyl cellulose, add appropriate amount of isopropanol and be mixed with 3% solution, standby as binder; Add binder in dabigatran etexilate, granulate; After the wet granules are dried, the granules are sized;
[0056] (2) Coating: take the remaining hydroxypropyl cellulose, add appropriate amount of isopropanol to prepare a 3% solution, add 2.2g talcum powder, stir evenly, and set aside; put the granules sized in step (1) in In a fluidized bed, coating is carried out.
[0057] Part II: Preparation of Tartaric Acid Coated Granules
[0058] (1) Granulation: get 0.9g hydroxypropyl cellulose, add appropriate amount of water and be m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com