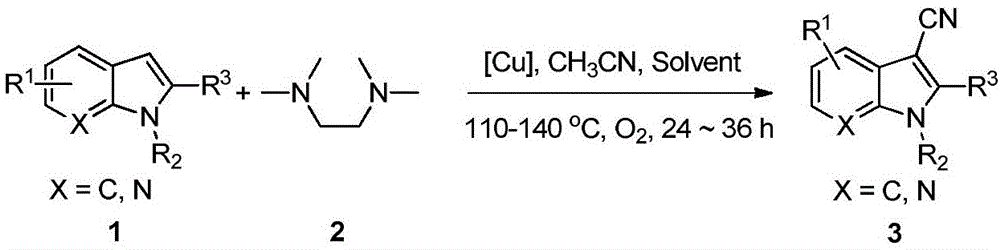
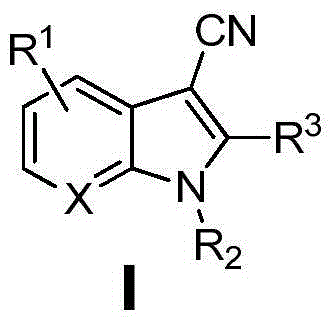
Synthesis method of copper-promoted 3-cyano-substituted-indole compound
A technology of indole compound and cyano group substitution, applied in organic chemistry and other directions, can solve the problems of easy poisoning, high price and high substrate requirements of Pd catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0023] Synthesis of 3-cyanindole
[0024] Add 0.2 mmol of indole, 0.3 mmol of cuprous chloride, 0.4 mmol of tetramethylethylenediamine, and then add 1 ml of a mixed solvent (the volume ratio of acetonitrile and DMAc is [7:3]) into the reaction vessel. Under an oxygen atmosphere, heat to 120°C, continue to stir for 24 hours, stop the reaction, cool to room temperature, extract with ethyl acetate, dry, and distill off the solvent under reduced pressure. The crude product is separated by column chromatography to obtain the target product with a yield of 41%. . 1 H NMR (400MHz, DMSO) δ12.24(s, 1H), 8.29(s, 1H), 7.67(d, J=7.6Hz, 1H), 7.59(d, J=8.0Hz, 1H), 7.31(t ,J=7.4Hz,1H),7.26(t,J=7.4Hz,1H).
Synthetic example 2
[0026] Synthesis of N-methylindole-3-cyano
[0027] Add 0.2mmol N-methylindole, 0.3mmol of cuprous chloride, 0.4mmol of tetramethylethylenediamine in the reaction vessel, then add 1ml of mixed solvent (the volume ratio of acetonitrile and DMAc is [7:3] ). Under an oxygen atmosphere, heat to 130°C, continue to stir for 24 hours, stop the reaction, cool to room temperature, extract with ethyl acetate, dry, and distill off the solvent under reduced pressure. The crude product is separated by column chromatography to obtain the target product with a yield of 93%. . 1 H NMR (400MHz, CDCl 3 )δ7.76(d, J=7.9Hz, 1H), 7.56(s, 1H), 7.40(d, J=8.0Hz, 1H), 7.36(t, J=7.4Hz, 1H), 7.30(t, J=7.3Hz,1H),3.85(s,3H).
Synthetic example 3
[0029] Synthesis of 1,2-Dimethylindole-3-cyano
[0030] In reaction vessel, add 0.2mmol 1,2-dimethylindole, the cuprous chloride of 0.32mmol, the tetramethylethylenediamine of 0.45mmol, then add 1ml mixed solvent (the volume ratio of acetonitrile and DMAc is [7 :3]). Under an oxygen atmosphere, heat to 130°C, continue to stir for 24 hours, stop the reaction, cool to room temperature, extract with ethyl acetate, dry, and distill off the solvent under reduced pressure. The crude product is separated by column chromatography to obtain the target product with a yield of 85%. . 1 H NMR (400MHz, CDCl 3 )δ7.67(d,J=7.2Hz,1H),7.32-7.24(m,3H),3.72(s,3H),2.59(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com