Ibuprofen microemulsion drug delivery system
A drug delivery system and microemulsion technology, which can be used in antipyretics, drug combinations, emulsion delivery, etc., and can solve problems such as common surfactant content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Preparation of Microemulsion Pseudo-ternary Phase Diagram
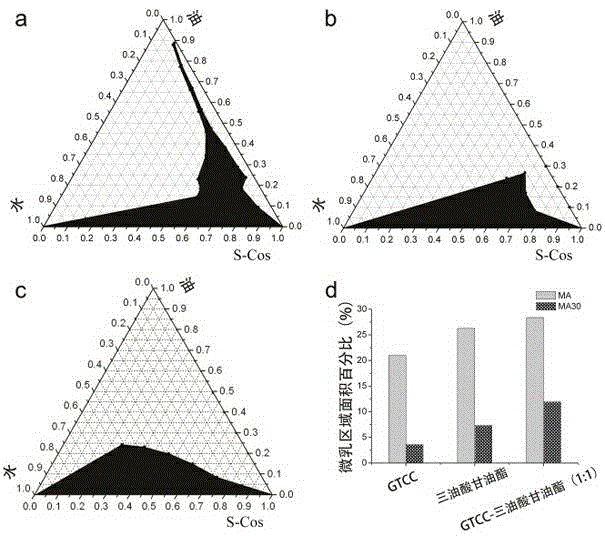
[0022]Weigh a certain amount of surfactant and co-surfactant, and mix thoroughly with a magnetic stirrer for 1 hour to obtain the total surfactant S-Cos. At 25°C, weigh S-Cos 1.8, 1.6, 1.4, 1.2, 1.0, 0.8, 0.6, 0.4, 0.2 g into vials, then add 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4 , 1.6, 1.8 g oil phase (the oil phase of a is caprylic triglyceride, the oil phase of b is triolein, the oil phase of c is caprylic triglyceride-triolein (1 :1)), mix well, slowly add distilled water dropwise to the mixture under magnetic stirring, take the appearance of "clear and transparent" as an indicator, record the amount of water added, according to the respective mass percentages of oil, total surfactant, and water at the critical point (w / w), draw a pseudo-ternary phase diagram, determine the microemulsion area, which is represented by a black area, and the results are shown in figure 1 a, 1b and 1c.
[0023] Pseudo...
Embodiment 2
[0024] Embodiment 2 The establishment of ibuprofen HPLC assay method
[0025] Chromatographic conditions: GraceSmart C18 column (250×4.6 mm, 5 μm), mobile phase methanol-pH3.0 phosphate buffer (60:40), flow rate 1 mL / min, injection volume 20 μL, detection wavelength λ = 220 nm, column temperature 35 o c.
[0026] Establishment of the standard curve: using methanol as a solvent, prepare a series of ibuprofen solutions with concentrations of 40, 20, 10, 5, 2.5, 1.2, and 0.6 μg / mL, and inject and analyze them respectively according to the predetermined chromatographic conditions. The peak area versus concentration was used as a standard curve. It can be seen that within the range of 0.6-40 μg / mL for ibuprofen, the peak area has a good linear correlation with the concentration (A=57.103C-5.6333, R 2 =0.9997).
Embodiment 3
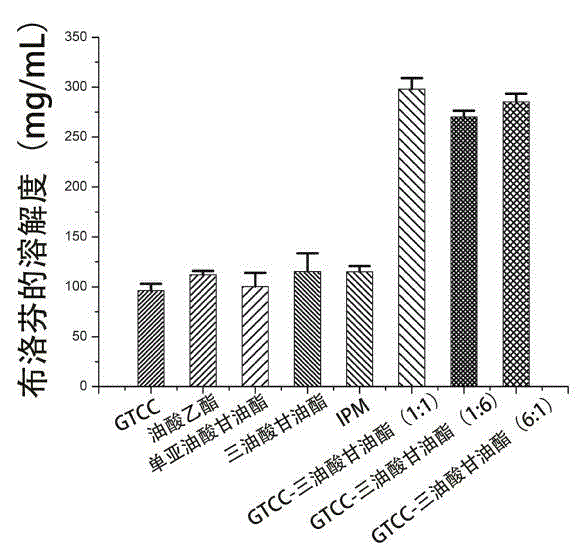
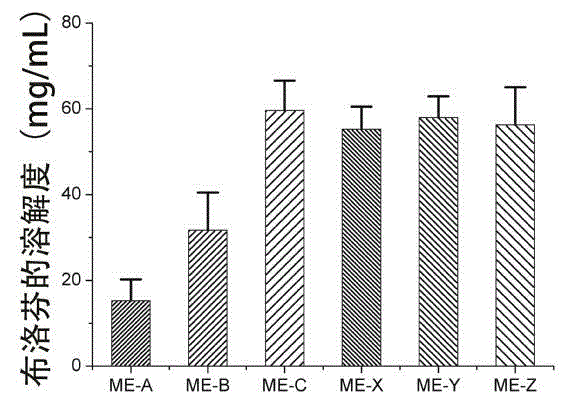
[0027] Embodiment 3 Solubility of ibuprofen in water
[0028] Take 2 mL of distilled water in a vial, make 3 parallel portions, add an appropriate amount of ibuprofen until precipitation occurs, vortex for 3-5 min to promote the dissolution of the drug in distilled water, and seal it. put in 25 o C in a constant temperature water bath shaker, 100 rpm for 72 h. After centrifugation at 15000 rpm for 5 min, the supernatant was taken, diluted appropriately with methanol, and the drug content was determined by HPLC. The solubility of ibuprofen in water was about 0.07 mg / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com