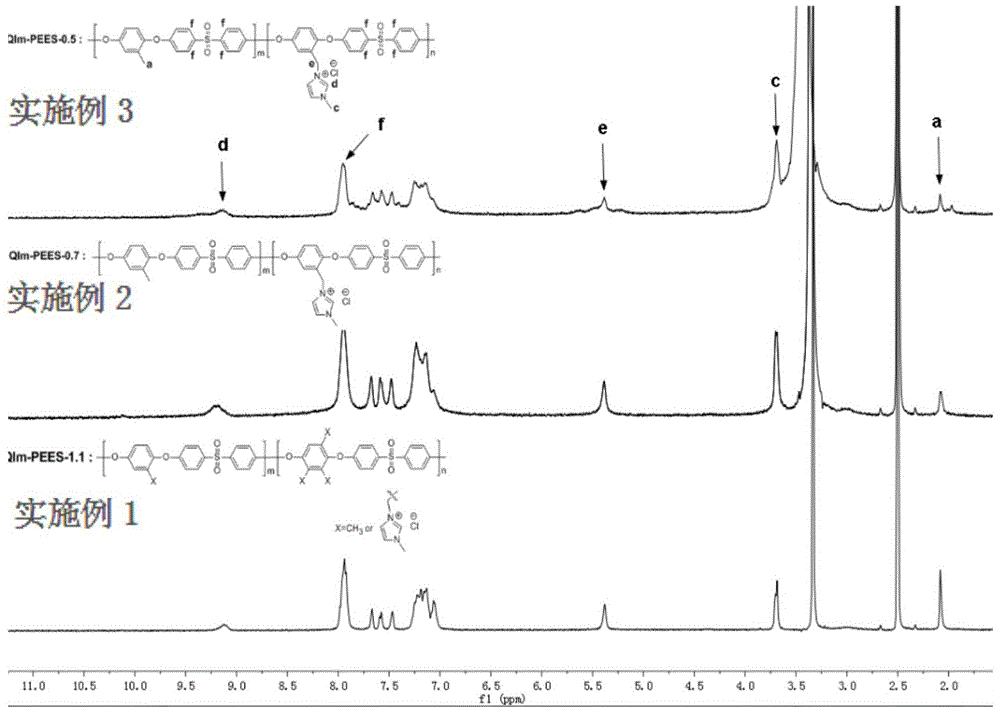
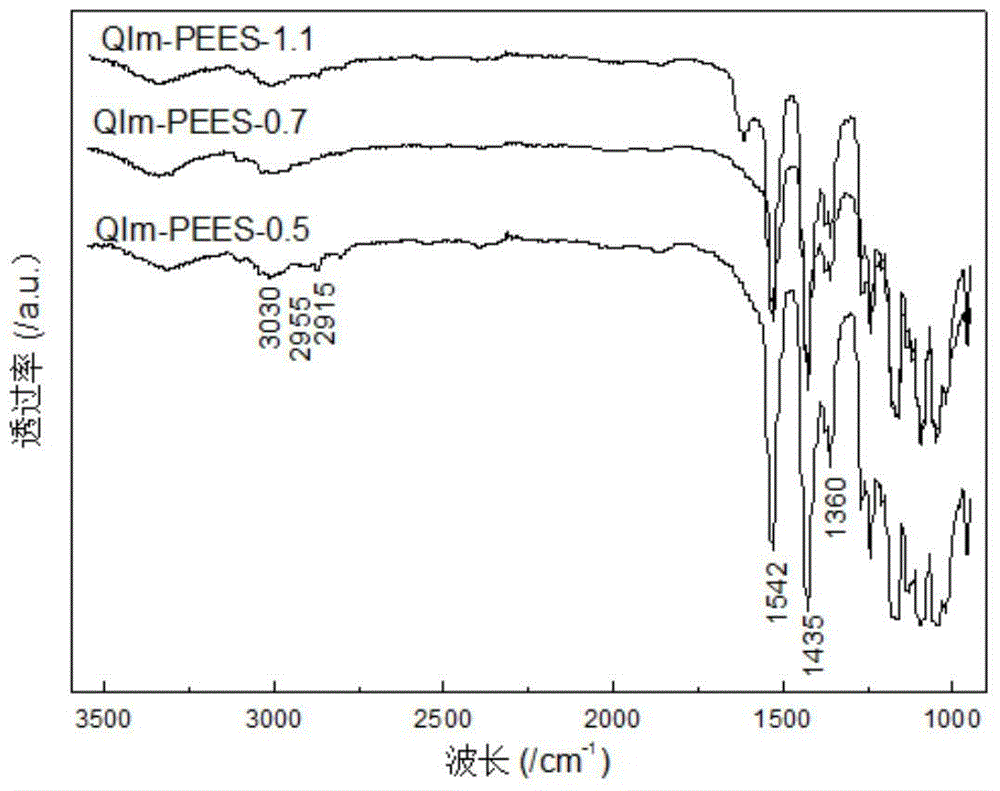
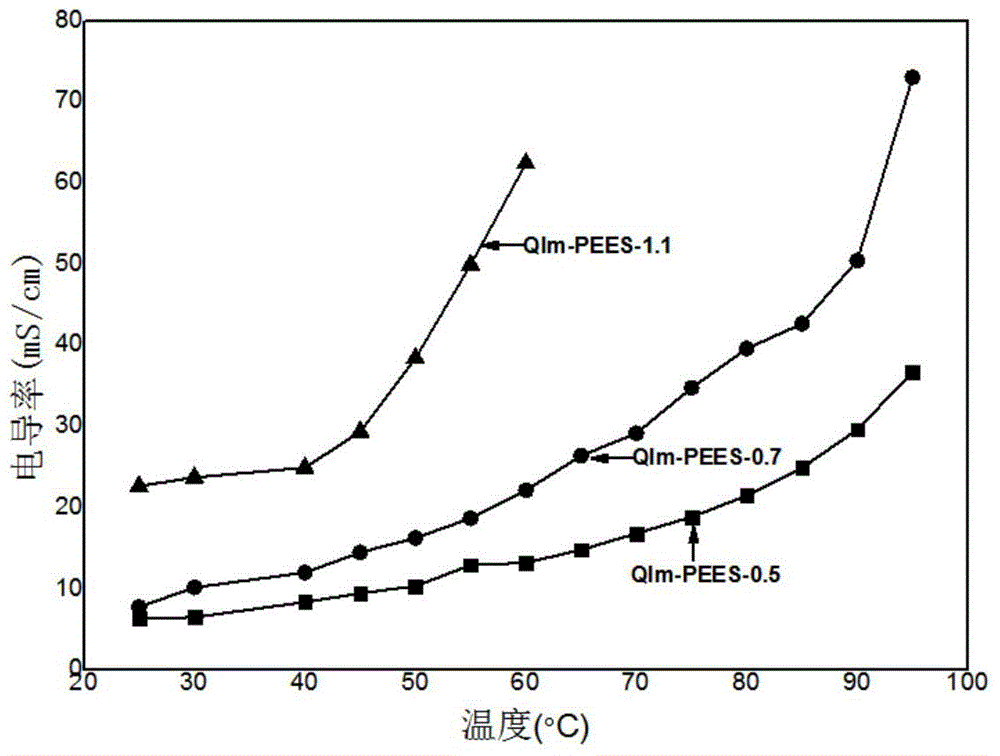
Imidazolium salt side group-containing poly(ether ether sulfone) anion-exchange membrane used for vanadium batteries, and preparation method thereof
An anion exchange membrane, polyether ether sulfone technology, applied in fuel cell parts, regeneration fuel cells and other directions, can solve the problems of high price, reduced vanadium ion permeability, no diaphragm material, etc., and achieves low cost and preparation process. Simple and easy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A kind of synthetic method of the polyether ether sulfone that side chain contains imidazole, comprises the following steps:
[0049] (1) In a round bottom flask, add 32.03g (0.258mmol) of 2-methylhydroquinone, 19.48g (0.128mmol) of 2,3,5-trimethylhydroquinone, 111g (0.386mol) ) of dichlorodiphenyl sulfone, 64.8g (0.468mol) of potassium carbonate, 100ml (86.6g) of toluene and 180ml (226.8g) of sulfolane solution, feed nitrogen into the reaction system and set insulation measures, at 150 ° C React for 2 hours to completely remove the solvent and the water in the reactant, and the color turns yellow.
[0050] (2) Raise the temperature to 170°C for 2 hours, remove toluene, and when no toluene evaporates, add the remaining solvent (300ml sulfolane, 378g), and the color becomes dark brown; then continue to heat up to 200°C, react for 5h, and the system Become viscous, stop the reaction.
[0051] (3) Pour the solution into deionized water while it is hot, stir vigorously to...
Embodiment 2
[0061] A kind of synthetic method of the polyether ether sulfone anion membrane containing imidazolium salt, comprises the following steps:
[0062] (1) In a round bottom flask, add 29.37g (0.193mol) of 2,3,5-trimethylhydroquinone, 55g (0.193mol) of dichlorodiphenyl sulfone, and 32.4g (0.234mol) of potassium carbonate , 55ml (47.63g) of toluene and 90ml (113.4g) of sulfolane solution, nitrogen into the reaction system, reacted at 150 ° C for 2h to fully remove the water in the reaction system, the color turned yellow.
[0063] (2) After the temperature rises to 170°C, remove the toluene, remove the insulation layer when the toluene solvent evaporates, add the remaining 150ml (189g) of sulfolane, the color becomes dark brown, then continue to heat up to 200°C, and react for 5 hours, The system became viscous and the reaction was terminated.
[0064] (3) The solution was poured into deionized water while it was hot, stirred and precipitated, and washed three times in succession...
Embodiment 3
[0074] A kind of synthetic method of the polyether ether sulfone anion membrane containing imidazolium salt, comprises the following steps:
[0075] (1) In a round bottom flask, add 23.96g (0.193molmmol) of 2-methylhydroquinone, 55g (0.193mol) of dichlorodiphenyl sulfone, 32.4g (0.234mol) of potassium carbonate, 55ml (47.6g ) of toluene and 90ml (113.4g) of sulfolane, nitrogen gas was passed into the reaction system, and the reaction was carried out at 150° C. for 2 hours to fully remove the water in the reaction system, and the color turned yellow.
[0076] (2) After the temperature rises to 170°C, remove the toluene, remove the insulation layer when the toluene solvent evaporates, add the remaining 150ml (189g) of sulfolane, the color becomes dark brown, then continue to heat up to 200°C, and react for 5 hours, The system became viscous and the reaction was terminated.
[0077] (3) The solution was poured into deionized water while it was hot, stirred and precipitated, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com