Preparation method of o-chlorotoluene
A technology of o-chlorotoluene and toluene, which is applied in the field of preparation of o-chlorotoluene, can solve the problems of inability to separate Lewis acid, be unfavorable for industrial production, and pollute the environment, and achieve the effects of easy control of the reaction, benefit for industrial production, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
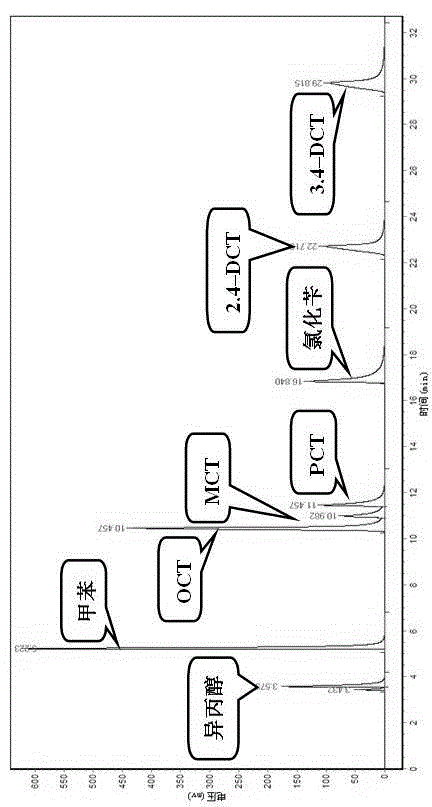
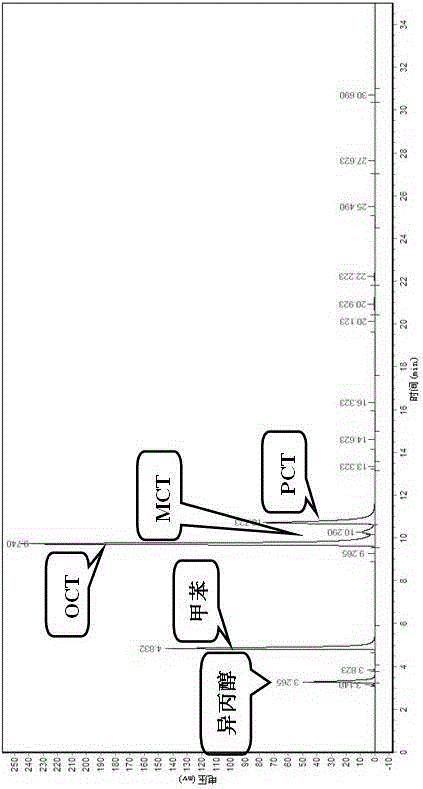
Image
Examples
Embodiment 1
[0025] (1) Ionic liquid [BMIM]Cl-2ZnCl 2 Preparation: under nitrogen protection, 1.3 mol of 1 – Methylimidazole and 1.4 mol of n-chlorobutane were stirred and mixed in a 500 mL three-neck flask with a condenser tube, and the temperature was raised to 80 °C under reflux for 48 h; the product was separated from the remaining unreacted liquid with a separatory funnel , the product was washed with ethyl acetate and washed three times; the crude product was rotary evaporated under vacuum at 60 °C to remove residual ethyl acetate, and dried in vacuum at 70 °C for 24 h, and the obtained light yellow liquid was [BMIM]Cl ionic liquid intermediate , stored in dry N 2 Reserve under atmosphere.
[0026] N 2 Atmosphere, 34.92 g (0.2mol) intermediate and 54.60 g (0.4mol) ZnCl 2 Add it to a three-necked flask, stir to disperse evenly, and react at 120 °C for 2 h.
[0027] (2) Toluene chlorination reaction step Add 1.0mol toluene to a 250 mL four-neck flask, add 0.03 mol [BMIM]Cl-2ZnCl ...
Embodiment 2
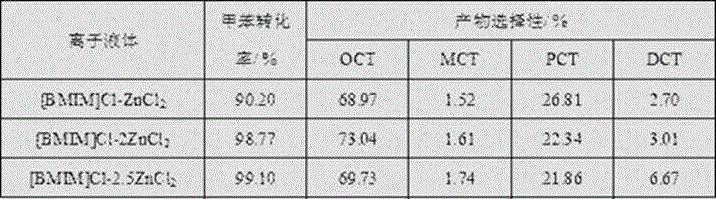
[0035] Adopt the same method of embodiment 1 to prepare [BMIM]Cl-2ZnCl 2 Ionic liquid, the process of toluene chlorination adopts the same method of embodiment 1, but changes [BMIM]Cl-2ZnCl 2 The dosage of ionic liquid is 0.01 mol, 0.05 mol, [BMIM]Cl-2ZnCl can be obtained 2 The impact of the amount of ionic liquid on the toluene chlorination process, as shown in Table 2:
[0036] Table 2 [BMIM]Cl-2ZnCl 2Effect of the dosage of ionic liquid on the chlorination reaction of toluene
[0037]
[0038] Table 2 shows the effect of catalyst dosage on toluene chlorination. When [BMIM]Cl-2ZnCl 2 When the ionic liquid is used as the catalyst, as the amount of catalyst increases from 1% to 3%, 5%, the conversion rate of toluene increases from 80.14% to 98.77%, 99.75%; the selectivity of o-chlorotoluene increases from 67.86% to 73.04%, Then it decreased to 68.58%.
[0039] As can be seen from Table 2, increasing the consumption of catalyst is conducive to the raising of toluen...
Embodiment 3
[0041] Adopt the same method of embodiment 1 to prepare [BMIM]Cl-2ZnCl 2 The process of ionic liquid and toluene chlorination reaction adopts the same method as in Example 1, but the reaction temperature is changed to 30°C, 50°C, and 90°C to obtain the influence of different reaction temperatures on the toluene chlorination process, as shown in Table 3:
[0042] Table 3 Effect of different reaction temperatures on the chlorination of toluene
[0043]
[0044] Table 3 reflects the influence of reaction temperature on the chlorination of toluene; as can be seen from Table 3, as the reaction temperature increases from 30 °C to 90 °C, the conversion rate of toluene increases from 78.25% to 99.84%; The selectivity increased first and then decreased. At 70 °C, the selectivity of o-chlorotoluene reached a maximum value of 73.04%; the selectivity of dichlorotoluene increased from 1.89% to 7.17%. increase and the selectivity of o-chlorotoluene, but also promote the generation o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com