Florfenicol included controlled-release preparation and its preparing method and application
A technology of florfenicol and controlled-release preparations, applied in the field of medicine, can solve problems such as poor clinical effects, achieve excellent sustained-release performance, prevent burst release, and improve bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
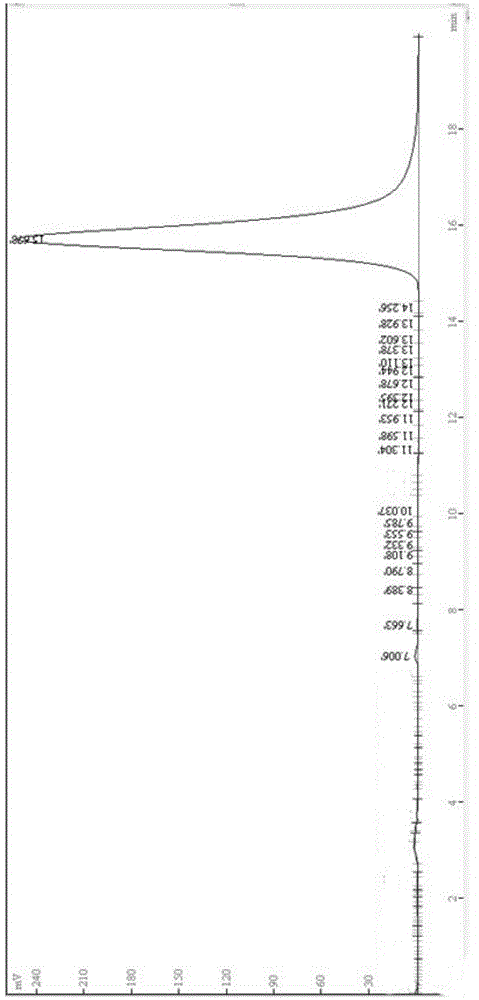
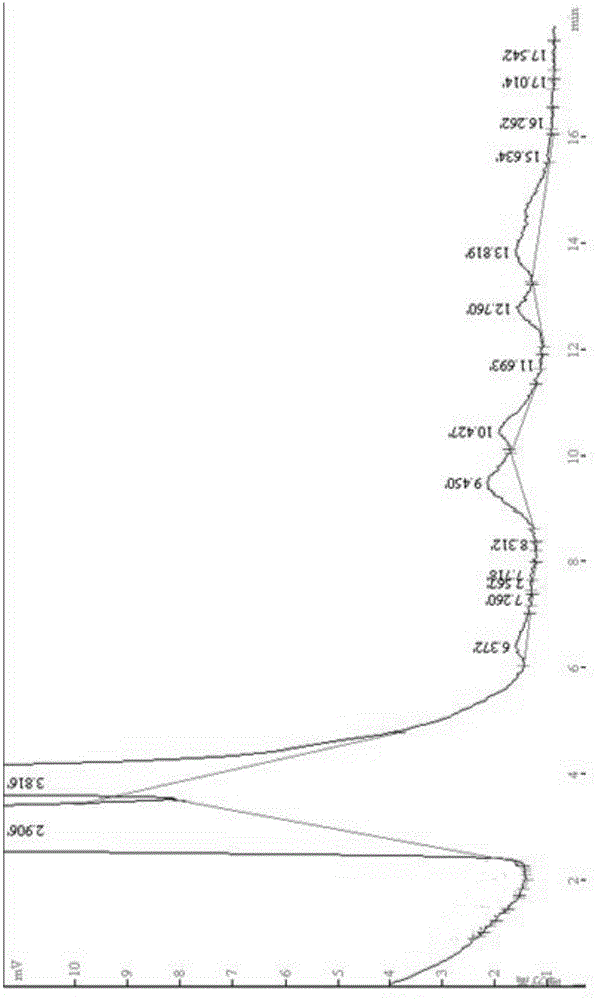
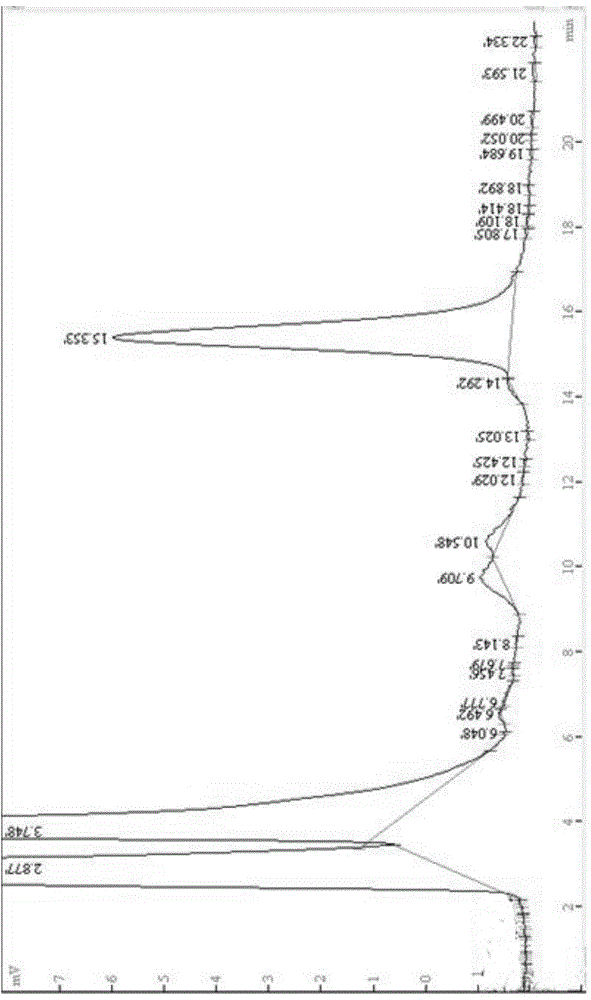
Image
Examples
Embodiment 1
[0050] The preparation of the florfenicol inclusion controlled-release preparation provided in this example is as follows:
[0051]
[0052] The specific preparation method is as follows:
[0053] (1) Take an appropriate amount of purified water and heat it to 80°C, add β-cyclodextrin and stir to dissolve, then add Florfenicol and stir for 30 minutes to form a Florfenicol inclusion solution;
[0054] (2) Adjust the spray dryer inlet air temperature to 160°C, the air outlet temperature to 80°C, and the pressure to 0.3MPa, and spray-dry the above-mentioned florfenicol inclusion solution to obtain the florfenicol soluble powder;
[0055](3) Take 66.25g of the above-mentioned Florfenicol soluble powder, microcrystalline cellulose, and sodium carboxymethylcellulose and mix them, add 26.47g of water, and mix them to make a soft material;
[0056] (4) Adjust the extrusion frequency of the extrusion spheronizer to 25 Hz, the spheronization frequency to 30 Hz, and the spheronizatio...
Embodiment 2
[0059] The preparation of the florfenicol inclusion controlled-release preparation provided in this example is as follows:
[0060]
[0061] The specific preparation method is as follows:
[0062] (1) Take an appropriate amount of purified water and heat it to 80°C, add β-cyclodextrin and stir to dissolve, then add Florfenicol and stir for 30 minutes to form a Florfenicol inclusion solution;
[0063] (2) Adjust the spray dryer inlet air temperature to 160°C, the air outlet temperature to 80°C, and the pressure to 0.3MPa, and spray-dry the above-mentioned florfenicol inclusion solution to obtain the florfenicol soluble powder;
[0064] (3) Take 66.25g of the above-mentioned Florfenicol soluble powder, microcrystalline cellulose, and sodium carboxymethylcellulose and mix them, add 26.47g of water, and mix them to make a soft material;
[0065] (4) Adjust the extrusion frequency of the extrusion spheronizer to 25 Hz, the spheronization frequency to 30 Hz, and the spheronizati...
Embodiment 3
[0068] The preparation of the florfenicol inclusion controlled-release preparation provided in this example is as follows:
[0069]
[0070] The specific preparation method is as follows:
[0071] (1) Take an appropriate amount of purified water and heat it to 80°C, add β-cyclodextrin and stir to dissolve, then add Florfenicol and stir for 30 minutes to form a Florfenicol inclusion solution;
[0072] (2) Adjust the spray dryer inlet air temperature to 160°C, the air outlet temperature to 80°C, and the pressure to 0.3MPa, and spray-dry the above-mentioned florfenicol inclusion solution to obtain the florfenicol soluble powder;
[0073] (3) Take 66.44g of the above-mentioned florfenicol soluble powder, microcrystalline cellulose, sodium carboxymethyl cellulose and mix, add 24.92g of water, and mix to make a soft material;
[0074] (4) Adjust the extrusion frequency of the extrusion spheronizer to 25 Hz, the spheronization frequency to 30 Hz, and the spheronization time to 4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com