Ether amine flotation agent synthesis method
A synthesis method and flotation agent technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of amino hydroxyl compounds, etc., can solve the problems of increasing the difficulty of product separation, high requirements for equipment operation, and prone to side reactions , to achieve the effects of low reaction process difficulty, simple and safe operation, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] A method for synthesizing etheramine flotation agents comprises the following steps: fully reacting fatty alcohol metal salts and haloalkylamines in a solvent under normal pressure.
[0027] Preferably, the fatty alcohol metal salt is C 5 ~C 12 Alkali metal salts of fatty alcohols, C 5 ~C 12 At least one of alkaline earth metal salts of fatty alcohols.
[0028] Preferably, said haloalkylamine is at least one of fluoroalkylamine, chloroalkylamine, bromoalkylamine, and iodoalkylamine; further preferably, said haloalkylamine The alkyl group is selected from one of ethyl, propyl, butyl, and pentyl.
[0029] Preferably, the solvent is fatty alcohol; more preferably, the fatty alcohol is C 5 ~C 12 fatty alcohol.
[0030] Preferably, the molar ratio of haloalkylamine to fatty alcohol is 1:4˜1:8.
[0031] Preferably, the molar ratio of haloalkylamine to fatty alcohol metal salt is 1:1˜1:1.5.
[0032] Preferably, the reaction temperature is 80-150° C.; the reaction time...
Embodiment 1
[0036] (1) Under normal pressure, add 0.1mol chloroethylamine hydrochloride, 0.3mol n-hexanol to the round bottom flask, gradually precipitate white needle-like crystals, react for 1h, filter, and collect the filtrate;
[0037] (2) Add 0.2mol n-hexanol and 0.1mol sodium hydride (4.80g, 50wt%) in a three-necked flask equipped with a condenser and a stirrer, and react for 30min;
[0038] (3) Add the filtrate of step (1) dropwise into the three-necked flask of step (2), heat up to 80°C, react for 2 hours, filter, and collect the filtrate to obtain hexyl ethyl ether amine solution.
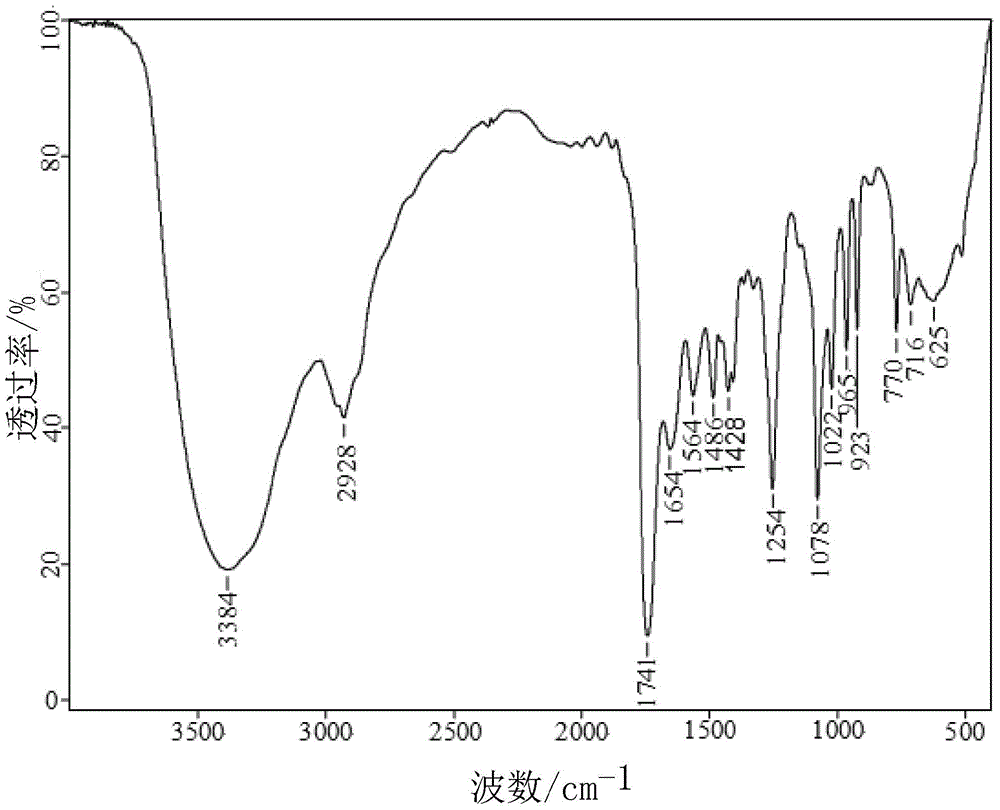
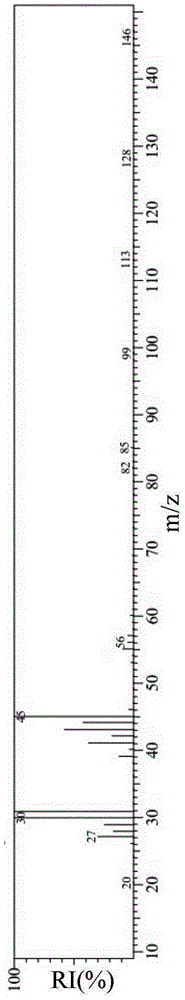
[0039] Such as figure 1 For the infrared spectrogram of the hexyl ethyl ether amine obtained in the present embodiment, figure 2 It is the mass spectrum of hexyl ethyl ether amine.
[0040] Correspondingly, the following two tables are the corresponding map analysis:
[0041] Table 1: The functional groups corresponding to the main absorption peaks in the infrared spectrum of hexyl ethyl ether a...
Embodiment 2
[0046] (1) Under normal pressure, add 0.1mol chloroethylamine hydrochloride, 0.25mol n-hexanol to the round bottom flask, gradually precipitate white needle-like crystals, react for 1h, filter, and collect the filtrate;
[0047] (2) Add 0.25mol n-hexanol and 0.13mol sodium hydride (6.24g, 50wt%) in a three-necked flask equipped with a condenser and a stirrer, and react for 30min;
[0048] (3) Add the filtrate of step (1) dropwise into the three-necked flask of step (2), raise the temperature to 80°C, react for 1.5h, filter, and collect the filtrate to obtain the hexyl ethyl ether amine solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com