Preparation method of aminoation reagent 2,4,6-trimethyl-benzenesulfonamide and benzenesulfonamide derivatives
A technology of trimethylbenzenesulfonamide and sulfonyl chloride derivatives is applied in the preparation of sulfonic acid amides, organic chemistry, etc., and can solve the problems of high requirements on reaction conditions, high cost of raw materials, complicated process operations, etc., and achieves easy availability of raw materials, The effect of high reactivity and controllable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of 2,4,6-trimethylbenzenesulfonamide
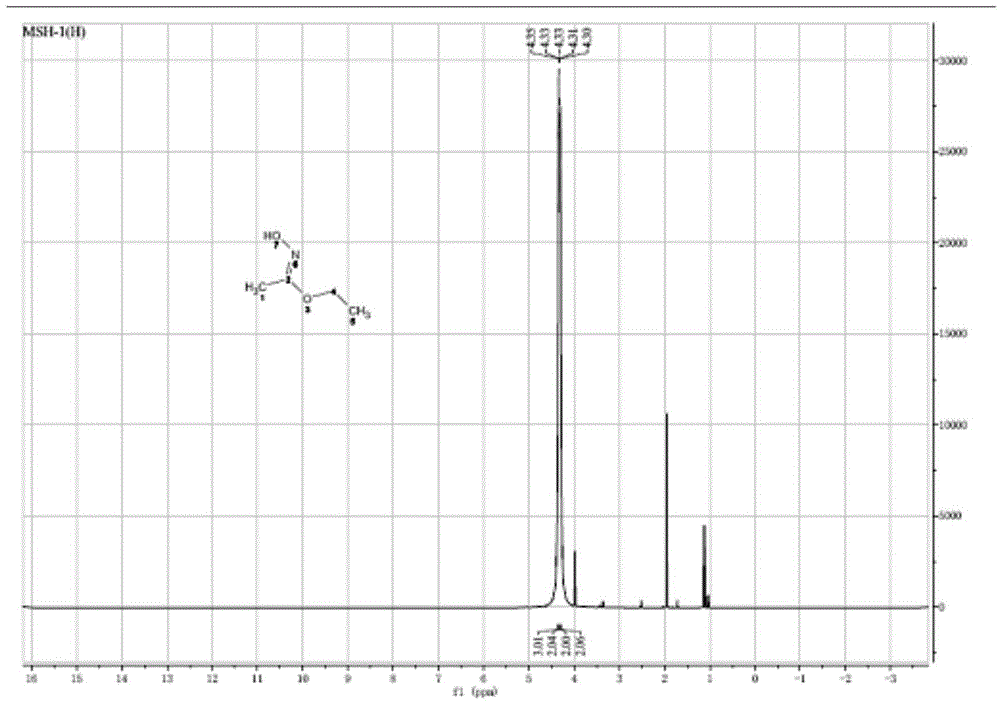
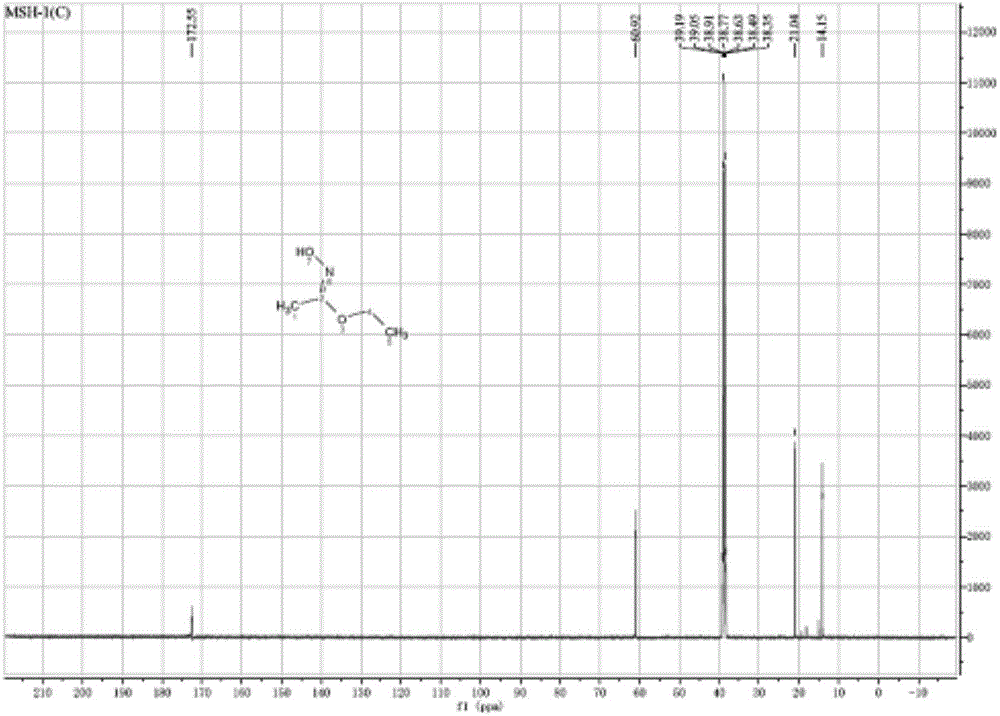
[0028] A. 18mL (0.2mol) of ethyl acetate and 21g (0.3mol) of hydroxylamine hydrochloride were stirred in 100mL ether solvent at 0°C for 45min to obtain a reaction solution; Continue stirring in the reaction solution; react at 0°C for 30 min, filter off the solid precipitate, extract the filtrate with 100 mL of ether, dry over anhydrous sodium sulfate, remove the ether under reduced pressure to obtain a white solid. figure 1 , figure 2 They are the proton NMR spectrum and carbon NMR spectrum of the white solid.
[0029] B. React the white solid obtained in step A with 10.5g trimethylbenzenesulfonyl chloride and 5g triethylamine in 15mL dimethylformamide at 0°C for 30min, pour it into ice-water mixture to precipitate a white precipitate (m.p.58°C ).
[0030] C. Dissolve the obtained white precipitate in 5 mL of dioxane solvent at 0° C., add 3 mL of perchloric acid with a mass concentration of 70% under stirr...
Embodiment 2
[0035] Example 2 Preparation of 2,4,6-trimethylbenzenesulfonamide
[0036] A, ethyl acetate 19kg and hydroxylamine hydrochloride 29kg were stirred in -5 DEG C 10L ether solvent for 30min to obtain a reaction solution; then 89.5kg of potassium carbonate was made into a concentration of 5mmol / L solution with distilled water and added to the reaction solution to continue stirring; React at -5°C for 45 min, filter off the solid precipitate, extract the filtrate with 2 L of ether, dry over anhydrous sodium sulfate, remove the ether under reduced pressure to obtain a white solid.
[0037] B. React the white solid obtained in step A with 23kg of trimethylbenzenesulfonyl chloride and 10kg of triethylamine in 28L of dimethylformamide at -3°C for 50min, and pour it into an ice-water mixture to precipitate a white precipitate (m.p.58°C ).
[0038] C. Dissolve the obtained white precipitate in 12L of dioxane solvent at -2°C, add 7.5L of perchloric acid with a mass concentration of 70% un...
Embodiment 3
[0040] Example 3 Preparation of 2,4,6-isopropylbenzenesulfonamide
[0041] A. Stir 19kg of ethyl acetate and 29kg of hydroxylamine hydrochloride in 10L ether solvent at 0°C for 60min to obtain a reaction solution; then add 86.5kg of potassium carbonate to a solution with a concentration of 2mmol / L in distilled water and add it to the reaction solution to continue stirring ;React at 0°C for 60min, filter off the solid precipitate, extract the filtrate with 2L ether, dry over anhydrous sodium sulfate, remove the ether under reduced pressure to obtain a white solid.
[0042] B. React the white solid obtained in step A with 45kg of triisopropylbenzenesulfonyl chloride and 10kg of triethylamine in 35L of dimethylformamide at 0°C for 60 minutes, and pour it into an ice-water mixture to precipitate a white precipitate (m.p.58°C ).
[0043] C. Dissolve the obtained white precipitate in 12L of dioxane solvent at 0°C, add 8L of perchloric acid with a mass concentration of 70% under stirr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com