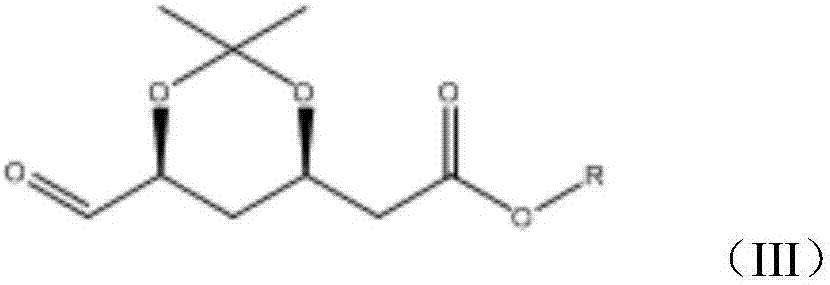
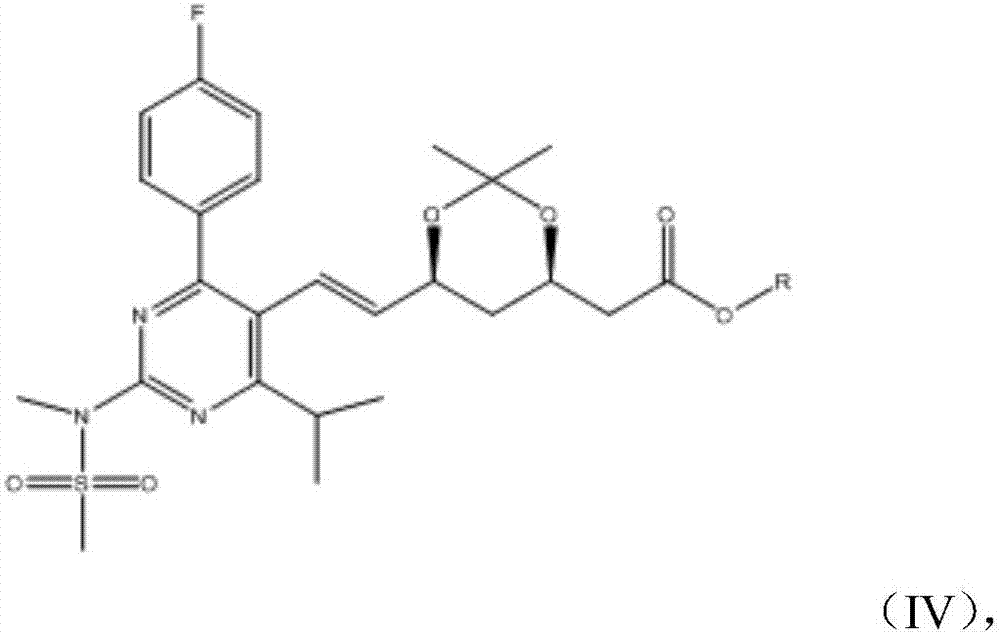
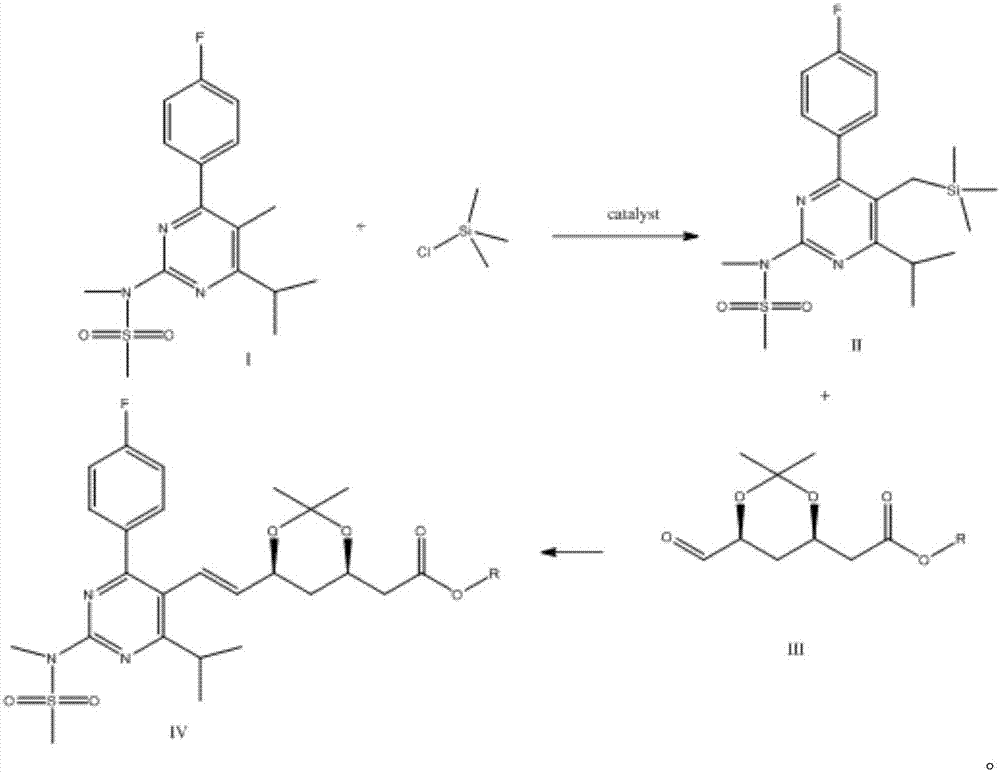
A kind of synthesis technique of the intermediate of synthetic rosuvastatin
A synthesis process and compound technology, applied in the direction of organic chemistry, etc., can solve the problems of by-products, three wastes, etc., and achieve the effects of less three wastes, low process cost, and less consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] In a 500ml four-necked bottle, a thermometer, a constant pressure dropping funnel and a magnetic stirrer were installed under N2 protection. At normal temperature, add 14 grams of compound I to the reaction flask, then add about 150 ml of THF, under nitrogen protection, stir to dissolve, then slowly cool down to -5 ~ -10 ° C, slowly start to drop n-butyllithium (2.5 N) n-hexane solution 30ml, the temperature is maintained at -10°C ~ 0°C, the time for dropping is about 45min, the temperature is raised to 0°C ~ 10°C, kept for 30min, the temperature is lowered to about -15°C, and 2, 2, 6 , 6-Tetramethylpiperidine 11g / THF 20ml mixed solution, the dropping time is about 30min, the temperature is controlled at -15±2℃, keep warm for 2 hours, slowly drop trimethylchlorosilane / THF (8g / 20ml ) for 3 hours (TLC detection of cyclohexane: ethyl acetate = 20:1) After the reaction is complete, pour it into 300ml of ice water, extract twice with 200ml of toluene, wash once w...
example 2
[0040] In a 500ml four-necked bottle, a thermometer, a constant pressure dropping funnel and a magnetic stirrer were installed under N2 protection. At normal temperature, add 14 grams of compound I to the reaction flask, then add about 150 ml of THF, under nitrogen protection, stir to dissolve, then slowly cool down to -5 ~ -10 ° C, slowly start to drop n-butyllithium (2.5 N) n-hexane solution 35ml, the temperature is maintained at -10°C ~ 0°C, the dropwise addition time is about 45min, the temperature is raised to 0°C ~ 10°C, kept for 30min, cooled to about -15°C, dropwise added 2, 2, 6 , 6-Tetramethylpiperidine 12.5g / THF 20ml mixed solution, the time for dropping is about 30min, the temperature is controlled at -15±2°C, keep warm for 2 hours, slowly add trimethylchlorosilane / THF (10g / 20ml) for 3 hours (TLC detection of cyclohexane: ethyl acetate = 20:1) After the reaction is complete, pour it into 300ml of ice water, use 200ml of toluene, extract twice, wash once with 100ml...
Embodiment 3
[0042]In a 500ml four-necked bottle, a thermometer, a constant pressure dropping funnel and a magnetic stirrer were installed under N2 protection. At room temperature, add 14 grams of compound I to the reaction bottle, then add about 150ml of THF, under nitrogen protection, stir to dissolve, then slowly cool down to -5 ~ -10°C, slowly start to drop 80ml of LDA (1M) solution , the temperature is maintained at -10°C to 0°C, the time for dropping is about 45 minutes, the temperature is raised to 0°C to 10°C, the temperature is kept for 30 minutes, and trimethylchlorosilane / THF (8.8g / 20ml) is slowly added dropwise and kept for 3 hours ( TLC detection of cyclohexane: ethyl acetate = 20:1) After the reaction is complete, pour it into 300ml of ice water, extract twice with 200ml of toluene, wash once with 100ml of saturated sodium bicarbonate, concentrate under reduced pressure to dryness, add 20ml of n-heptane and 1ml of ethyl acetate were heated to dissolve, slowly cooled to -5°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com