Targeting STAT3 inhibitor and application thereof
A technology of targeting activity and thiophene, applied in the field of medicine, can solve the problems of less research, low bioavailability, complex structure of STAT3 inhibitors, etc., and achieve the effect of good anti-tumor activity and good physicochemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
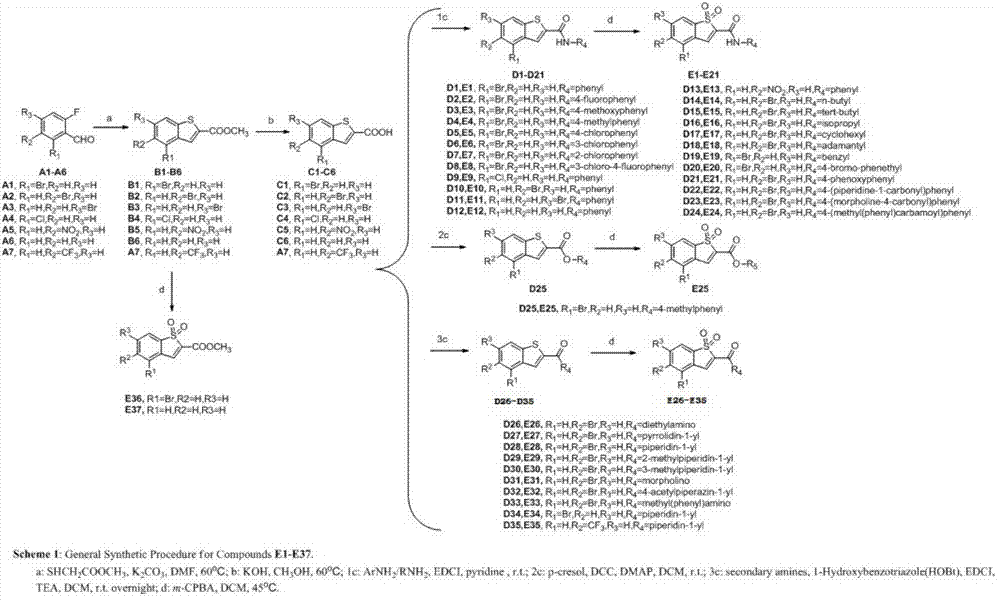
[0071] Embodiment 1 Preparation of benzothiophene 1,1-dioxy derivatives E1-E44
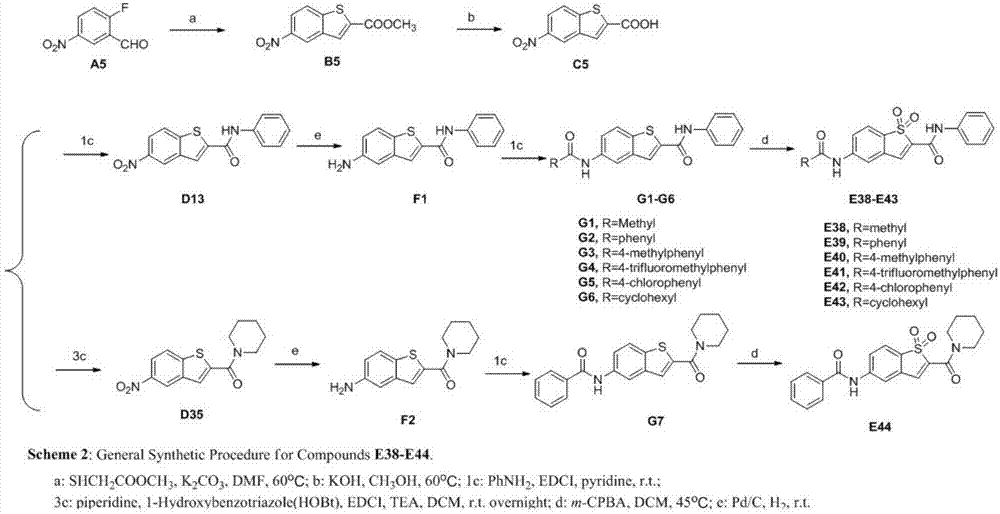
[0072] Synthetic routes of benzothiophene 1,1-dioxo derivatives E1-E44 (see figure 1 , figure 2 ):
[0073] (1) Preparation of Intermediate B1-B7:
[0074] Compound A1-A7 (9.90mmol) was dissolved in 20mL of DMF, adding K 2 CO 3 (5.47g, 39.6mmol), methyl thioglycolate (10.9mmol, 0.97mL), the reaction system was placed in an oil bath at 60°C and stirred for about 3h. After the reaction was completed, 50 mL of ice water was added to the reaction system, and a white solid was precipitated immediately, filtered under reduced pressure, and the filter cake was dried under an infrared lamp to obtain white solids B1-B7, yield: 67%-85%.
[0075] (2) Preparation of intermediate C1-C7:
[0076] Compounds B1-B7 (1.1 mmol) were suspended in 10 mL of aqueous methanol (80%), potassium hydroxide (0.300 g, 5.4 mmol) was added to the reaction system, and stirred at room temperature for about 16 h. After the ...
Embodiment 2
[0263] Example 2 Antitumor Activity Experiment of Benzothiophene 1,1-Dioxy Derivatives E1-E44
[0264] Example 1 provides the synthesis route of benzothiophene 1,1-dioxy derivatives E1-E44, and the following biological tests are carried out after the H NMR and C NMR structures are confirmed to be correct. The following are p-benzothiophene 1,1-dioxy derivatives E1-E44 in breast cancer cells (MDA-MB-231, MDA-MB-435S, MCF-7), prostate cancer cells (Du145), pancreatic cancer cells ( PANC-1), non-small cell lung cancer cells (A549) to evaluate the anti-proliferation effect (ie MTT test):
[0265] (1) Cytotoxicity test (MTT) of benzothiophene 1,1-dioxy derivatives E1-E44 on breast cancer cells (MDA-MB-231) and prostate cancer cells (DU145)
[0266] MTT test: With Stattic as a positive control, the MTT method was used to determine the in vitro cytotoxicity test of the compound on breast cancer cells (MDA-MB-231) and prostate cancer cells (DU145). The compound was made into a mothe...
Embodiment 3
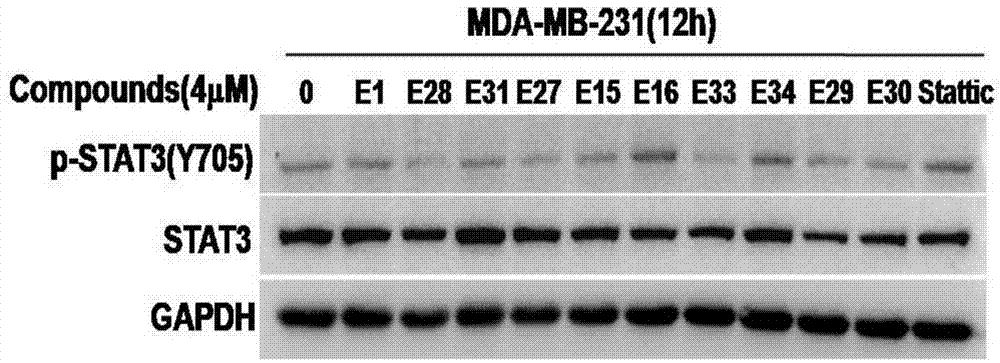
[0278] Example 3 Effect of Benzothiophene 1,1-Dioxy Derivatives E1-E44 on Phosphorylation of STAT3 in Breast Cancer Cells
[0279] Western blot: breast cancer cells MDA-MB-231 and MDA-MB-435S growing in the logarithmic phase were respectively mixed with 1.5×10 6 The density of the dish is spread at 10cm 2 in the petri dish. After 12 hours of wall attachment, compounds E1, E15, E16, E27, E28, E29, E30, E31, E33, and E34 were added at a concentration of 4 μM, and Stattic was used as a positive control. After 12 hours of drug treatment, the cells were collected in a 1.5mL EP tube, an appropriate amount of cell lysate was added, and lysed on ice for 30 minutes, followed by 1.48×10 4 Centrifuge at r / min, take the supernatant, and measure the protein concentration. Calculate the protein concentration of the sample according to the standard protein concentration, add the corresponding 5×SDS protein loading buffer, and cook the protein at 100°C for 5 minutes. Prepare SDS-PAGE gel ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com