Preparation method and application of protein chip based on serological detection of syphilis
A protein chip and syphilis technology, applied in the field of biotechnology detection, can solve the problems of inability to make correct diagnosis alone, low sensitivity and specificity, and inability to distinguish recent or previous syphilis infection and treatment or no treatment, and achieve important Clinical value and social benefit, high specificity, high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1, gold foil chip surface chemical modification
[0085] Step 1: Cleaning of Gold Foil Chips
[0086] Prepare TL1 solution (H 2 O: H 2 o 2 : NH 3 ·H 2 O=5:1:1, volume ratio) into a stainless steel box, put the gold foil chip into the box, bathe in 82°C water for 6 minutes, rinse with deionized water 4-5 times, ethanol twice, 3 minutes each time; air dry with nitrogen, Store dry.
[0087] Step 2: Chemically modify the surface of the cleaned gold foil chip to obtain a solid phase carrier
[0088] Spot the modification solution DSU on the washed gold foil chip (0.85 μl per spot), and incubate for 2 h in a humid chamber at room temperature in the dark. Wash 5 times with acetone, 4 minutes each time; then wash 3 times with PBS (PH7.4) solution, 2 minutes each time. Dry with nitrogen gas to obtain a solid phase carrier, which is ready for use.
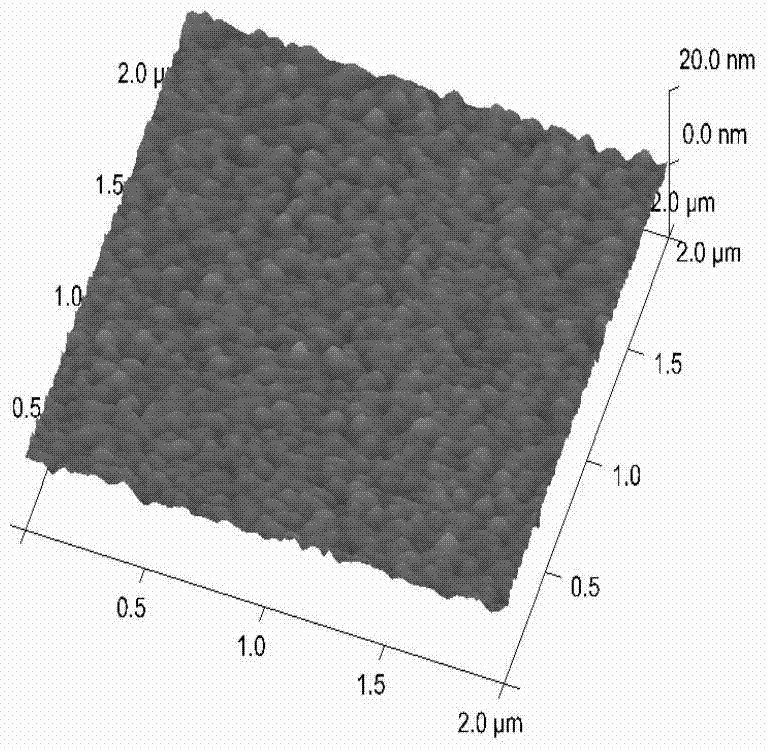
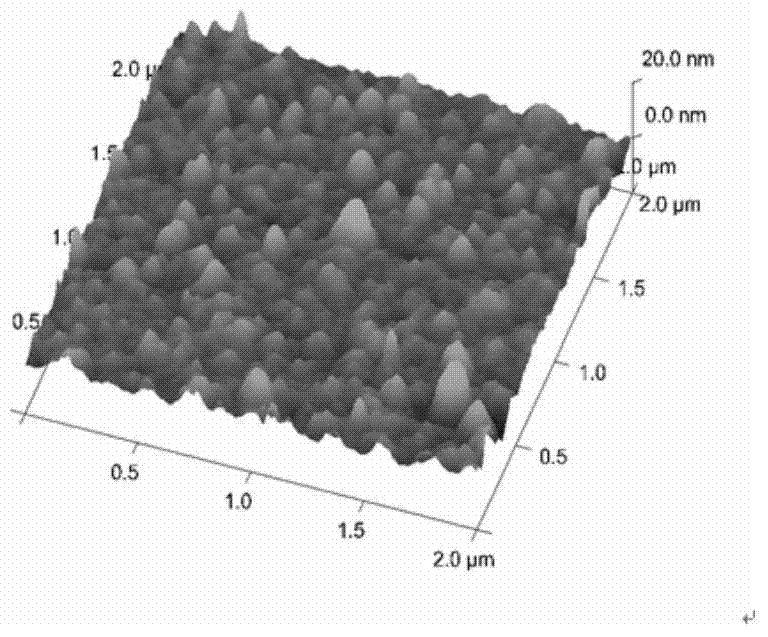
[0089] figure 2 with image 3 The characterization of gold foil chip before and after DSU modification was ob...
Embodiment 2
[0090] Embodiment 2, quality control experiment
[0091] Preparation of incubation solution 1: Dissolve Treponema pallidum recombinant antigen rTpN15-17-47 in PBST-BSA solution, and configure a concentration gradient of 200 μg / mL, 100 μg / mL, 50 μg / mL, 25 μg / mL, 12.5 μg / mL, 6.25 μg / mL, 3.13μg / mL, 1.56μg / mL, 0.78μg / mL, 0.39μg / mL, 0.19μg / mL solution.
[0092] Prepare incubation solution 2: dissolve the polyclonal rabbit anti-syphilis antigen (rTpN15-17-47) IgG antibody in PBST-BSA solution, and configure the antibody concentration gradient to be 200 μg / mL, 100 μg / mL, 50 μg / mL, 25 μg / mL, 12.5μg / mL, 6.25μg / mL, 3.13μg / mL, 1.56μg / mL, 0.78μg / mL, 0.39μg / mL, 0.19μg / mL solution.
[0093] Prepare incubation solution 3: Dissolve Cy3-labeled goat anti-rabbit IgG antibody in PBST-BSA solution, and configure the fluorescent secondary antibody concentration gradient as 5 μg / mL, 2.5 μg / mL, 1.25 μg / mL, 0.625 μg / mL, 0.313 μg / mL mL, 0.156μg / mL, 0.078.μg / mL, 0.039μg / mL, 0.019μg / mL solution.
[...
Embodiment 3
[0116] Embodiment 3, repeatability experiment
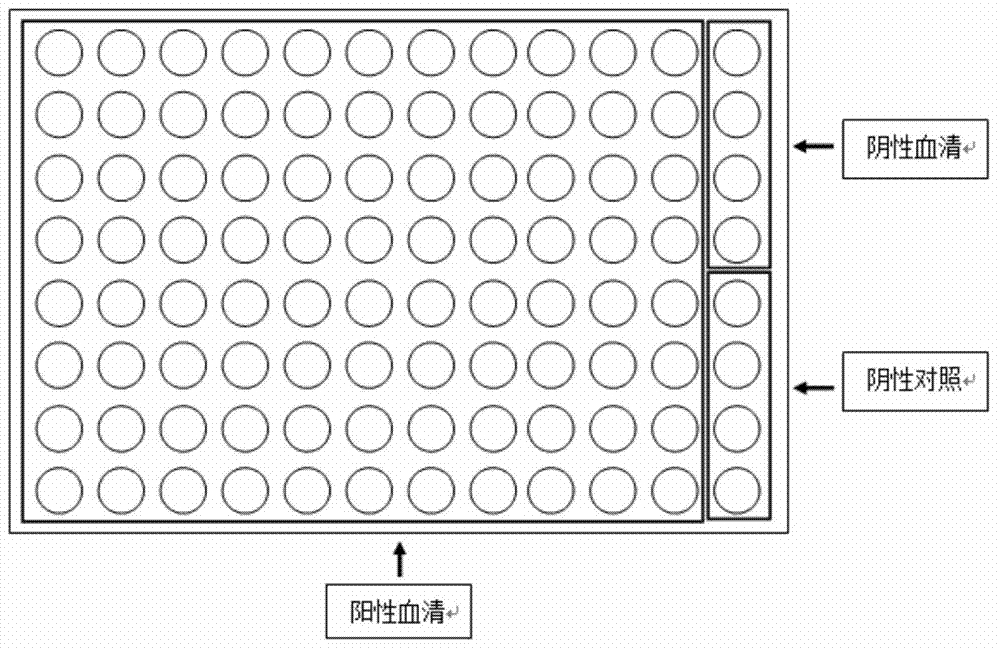
[0117] Each 32 wells were regarded as one group, and there were three groups in total. According to the method steps of Examples 1 to 3, the concentration of Treponema pallidum recombinant antigen rTpN15-17-47 and the concentration of 50 μg / mL of rabbit anti-Treponema pallidum antigen IgG were incubated. Antibody and Cy3-labeled goat anti-rabbit IgG antibody at a concentration of 2.5 μg / mL. Repeatability experiment results, as shown in Table 1 and Figure 8 shown.
[0118] Table 1
[0119]
[0120] As shown in Table 1, SD is the standard deviation, and CV is the coefficient of variation. These two indicators can reflect the stability of the protein chip in the detection of anti-rTpN15-17-47IgG antibody and there will be no large deviation.
[0121] Such as Figure 8 Shown: The fluorescence results of the three groups are not limited by different sample wells, with good repeatability and high stability.
[0122] From Table...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com