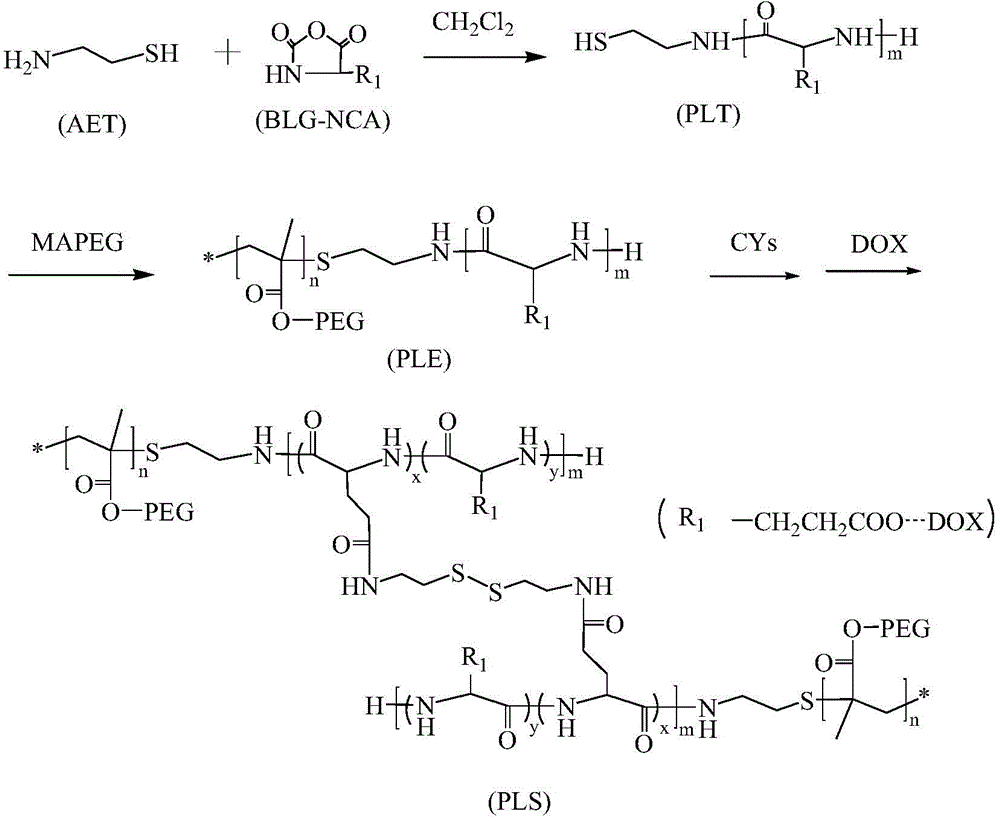
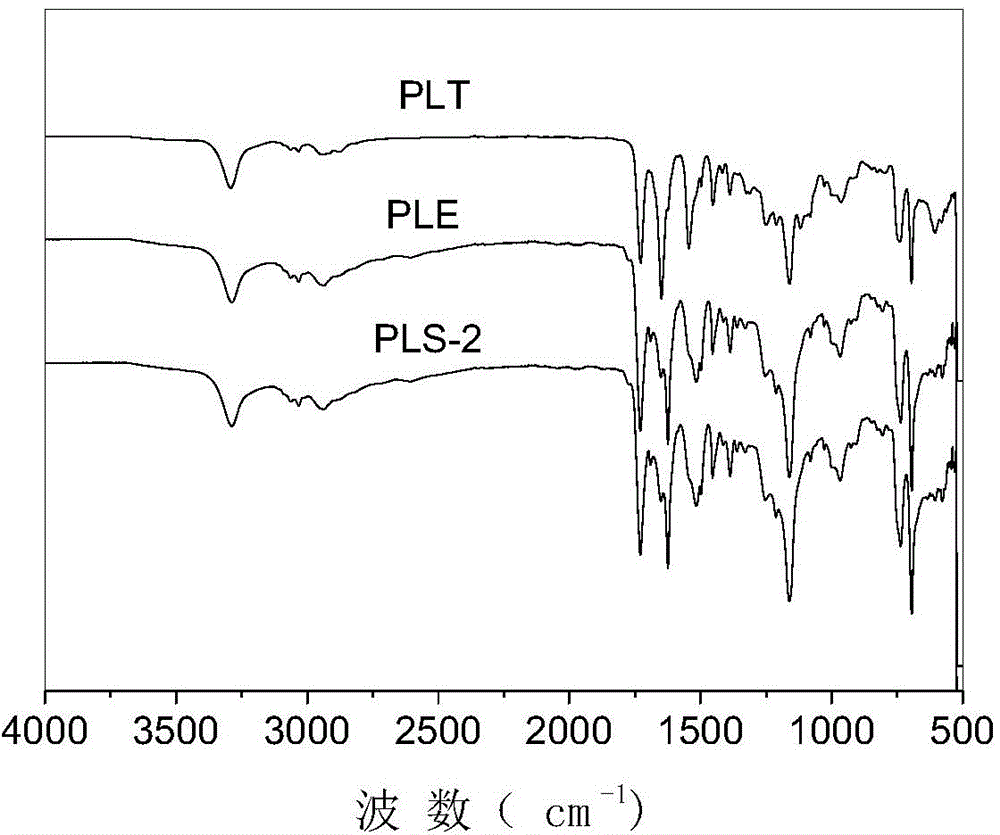
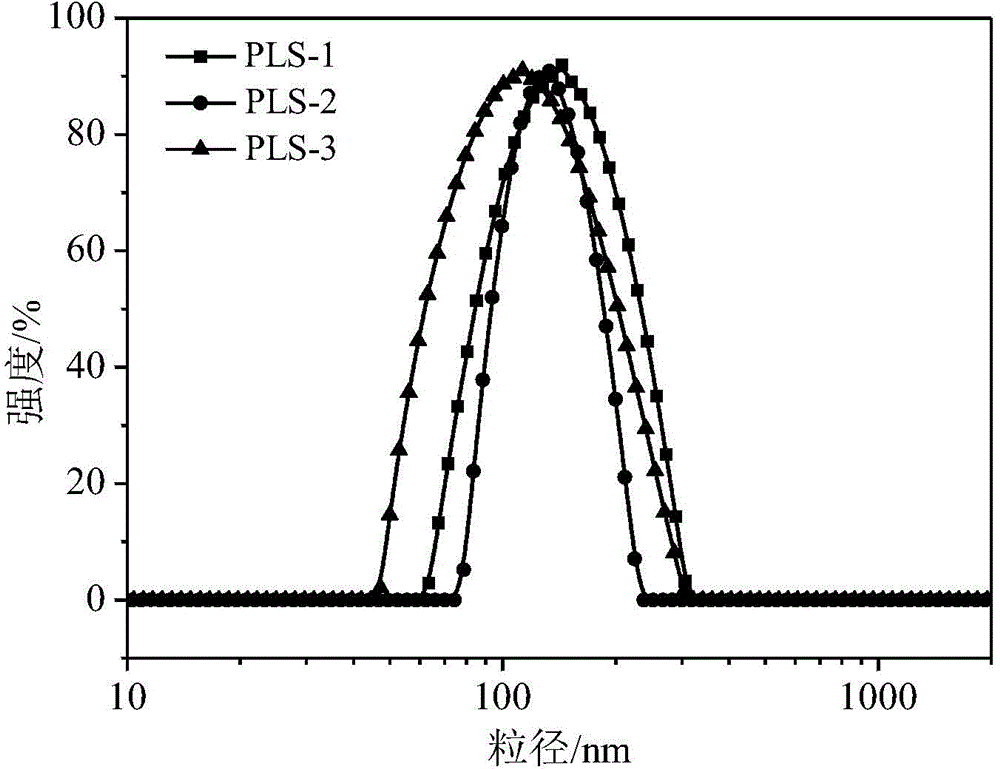
Preparation of poly(L-glutamic acid)-b-polyethylene glycol medicine carrying nano micelle
A drug-loaded nano-polyethylene glycol technology, which is applied to medical preparations containing non-active ingredients, medical preparations containing active ingredients, and anti-tumor drugs, etc., to achieve prolonged maintenance of drug efficacy, significant reduction responsiveness, and easy absorption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Preparation of sulfhydryl-terminated poly(L-glutamic acid-γ-benzyl ester)
[0019] In a three-necked flask replaced with nitrogen beforehand, mercaptoethylamine (AET) and BLG-NCA with a molar feed ratio of 1:40 were sequentially added, and the two were dissolved in methylene chloride solvent to make the total concentration of reactants 10w%. Stir magnetically for 72 hours at room temperature in a nitrogen atmosphere, concentrate the reaction solution, and drop it into excess butyl acetate, ice bath for half an hour to obtain a flocculent precipitate, centrifuge to obtain a pale yellow precipitate, and vacuum dry at 35°C to obtain terminal sulfhydryl groups Poly(L-glutamic acid-γ-benzyl ester), referred to as PLT.
Embodiment 2
[0020] Example 2: Preparation of poly(L-glutamic acid-γ-benzyl ester)-b-polyethylene glycol
[0021] PLT and polyethylene glycol methacrylate (MAPEG) are co-dissolved in DMF solution. The molecular weight of polyethylene glycol methacrylate is M n =475g / mol, the molar ratio of PLT to polyethylene glycol methacrylate is 1:1~1:1.5, the two are dissolved in DMF solvent, the total concentration of reactants is 15w%, and the total weight of reactants is 1 % AIBN is the initiator. Free radical polymerization is carried out at 60℃. After 24 hours, the reaction solution is added dropwise to ultrapure water to obtain a milky white turbid liquid, which is transferred to a dialysis bag with a molecular weight cut-off of 3500 for 72 hours. Change the water every 12 hours, and finally freeze-dry the product to obtain a pale yellow solid poly(L-glutamic acid-γ-benzyl ester)-b-polyethylene glycol, abbreviation: PLE;
Embodiment 3
[0022] Example 3: Crosslinking of micelles
[0023] The PLE obtained in Example 2 and cystamine were mixed and added to the DMF solvent. The cystamine and PLE were fed at a molar ratio of 1:1, 2:1, 4:1, and the total concentration of the two dissolved in the DMF solvent was 12w. %, magnetically stirred at 30℃ for 5h and then cooled to room temperature, take the reaction solution, add ultrapure water dropwise to it and stir until there is obvious Tyndall effect, dialyze with a dialysis bag with molecular weight cut off of 3500 for 72h and freeze-dry , Then prepare a micelle solution with a concentration of 0.8w% for use; prepare a series of crosslinked micelles with different crosslinking degrees according to the above ratio. See synthetic route figure 1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com