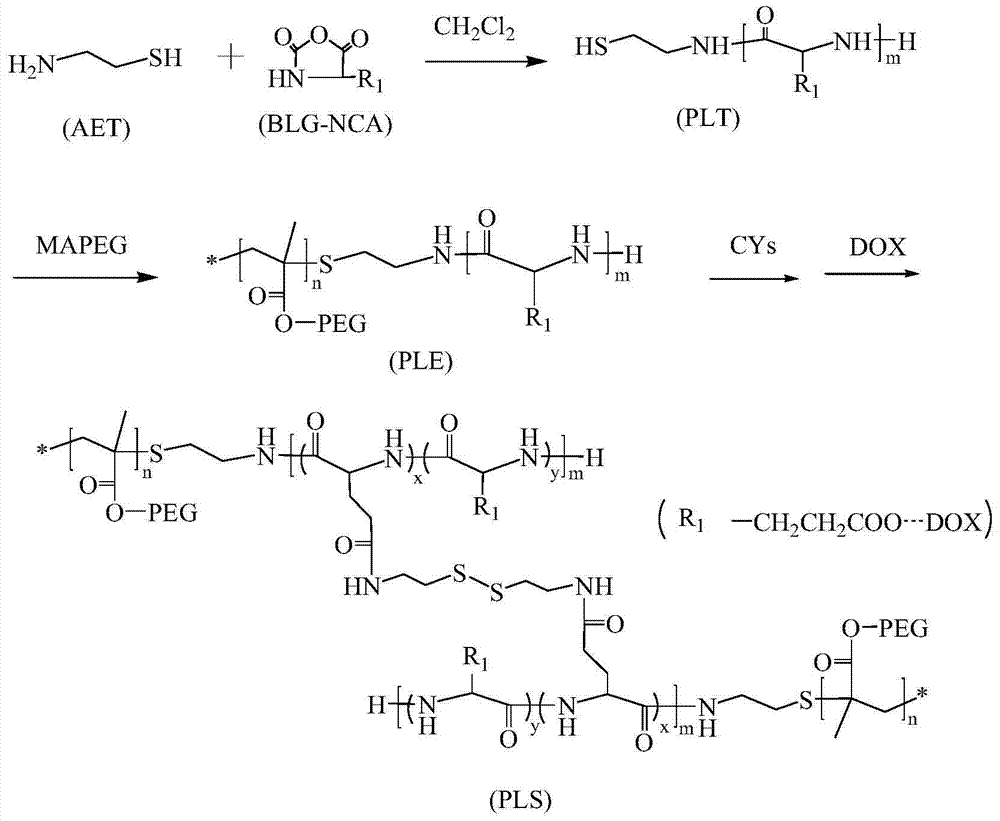
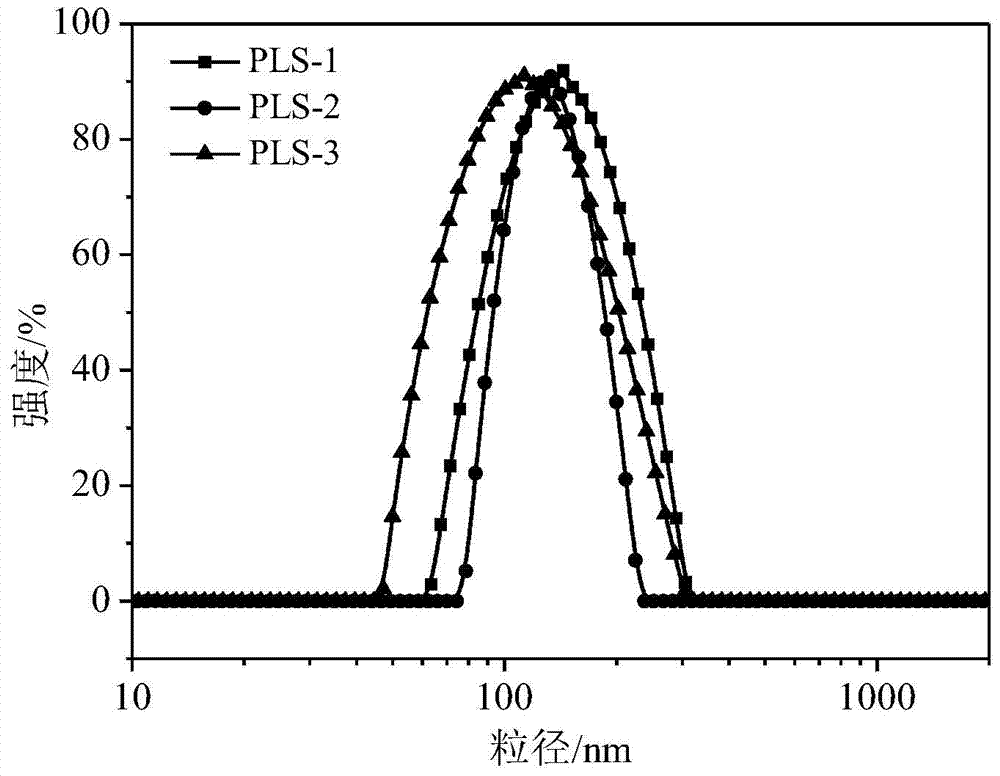
Preparation of a poly(l-glutamic acid)-b-polyethylene glycol drug-loaded nanomicelle
A drug-loaded nano-polyethylene glycol technology, which is applied to non-active ingredient medical preparations, active ingredient-containing medical preparations, anti-tumor drugs, etc., to enhance drug efficacy, strong salt resistance and dilution resistance , the effect of reducing the probability of drug leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: Preparation of thiol-terminated poly(L-glutamic acid-γ-benzyl ester)
[0019] Adding mercaptoethylamine (AET) and BLG-NCA with a molar feed ratio of 1:40 in the three-necked flask replaced with nitrogen in advance, the two are dissolved in methylene chloride solvent, so that the total concentration of reactants is 10w%, Stir magnetically at room temperature in a nitrogen atmosphere for 72 hours, concentrate the reaction solution, and drop it into excess butyl acetate, ice-bath for half an hour to obtain a flocculent precipitate, centrifuge to obtain a light yellow precipitate, and vacuum-dry at 35°C to obtain a terminal mercapto group Poly(L-glutamic acid-γ-benzyl ester), referred to as PLT.
Embodiment 2
[0020] Example 2: Preparation of poly(L-glutamic acid-γ-benzyl ester)-b-polyethylene glycol
[0021] PLT and polyethylene glycol methacrylate (MAPEG) are co-dissolved in the DMF solution, and the molecular weight of the polyethylene glycol methacrylate used is M n =475g / mol, the molar ratio of PLT to polyethylene glycol methacrylate is 1:1~1:1.5, the two are dissolved in DMF solvent, the total concentration of the reactants is 15w%, and the total weight of the reactants is 1 % of AIBN as the initiator, free radical polymerization was carried out at 60°C, and after 24 hours, the reaction solution was added dropwise into ultrapure water to obtain a milky white turbid liquid, which was transferred to a dialysis bag with a molecular weight cut-off of 3500 for dialysis for 72 hours. Change the water every 12 hours, and finally freeze-dry the product to obtain light yellow solid poly(L-glutamic acid-γ-benzyl ester)-b-polyethylene glycol, referred to as PLE;
Embodiment 3
[0022] Example 3: Cross-linking of micelles
[0023] Mix PLE obtained in Example 2 and cystamine into the DMF solvent, cystamine and PLE are fed in molar ratios of 1:1, 2:1, and 4:1 respectively, and the total concentration of the two dissolved in the DMF solvent is 12w %, stirred magnetically at 30°C for 5 hours, cooled to room temperature, took the reaction solution, added dropwise into ultrapure water and stirred until Tyndall effect was evident, dialyzed with a dialysis bag with a molecular weight cut-off of 3500 for 72 hours, and then freeze-dried , and then prepared a micellar solution with a concentration of 0.8w% for later use; a series of cross-linked micelles with different cross-linking degrees were prepared according to the above ratio. Synthetic route see figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com