Amphiphilic graft copolymer based on hyaluronic acid, and preparation method and application of amphiphilic graft copolymer
A technology of graft copolymer and hyaluronic acid, which is applied in the direction of application, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of insufficient tissue adhesion, slow wound healing, and insufficient water absorption capacity and other issues, to achieve good biocompatibility, easy structure modification, and large water absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
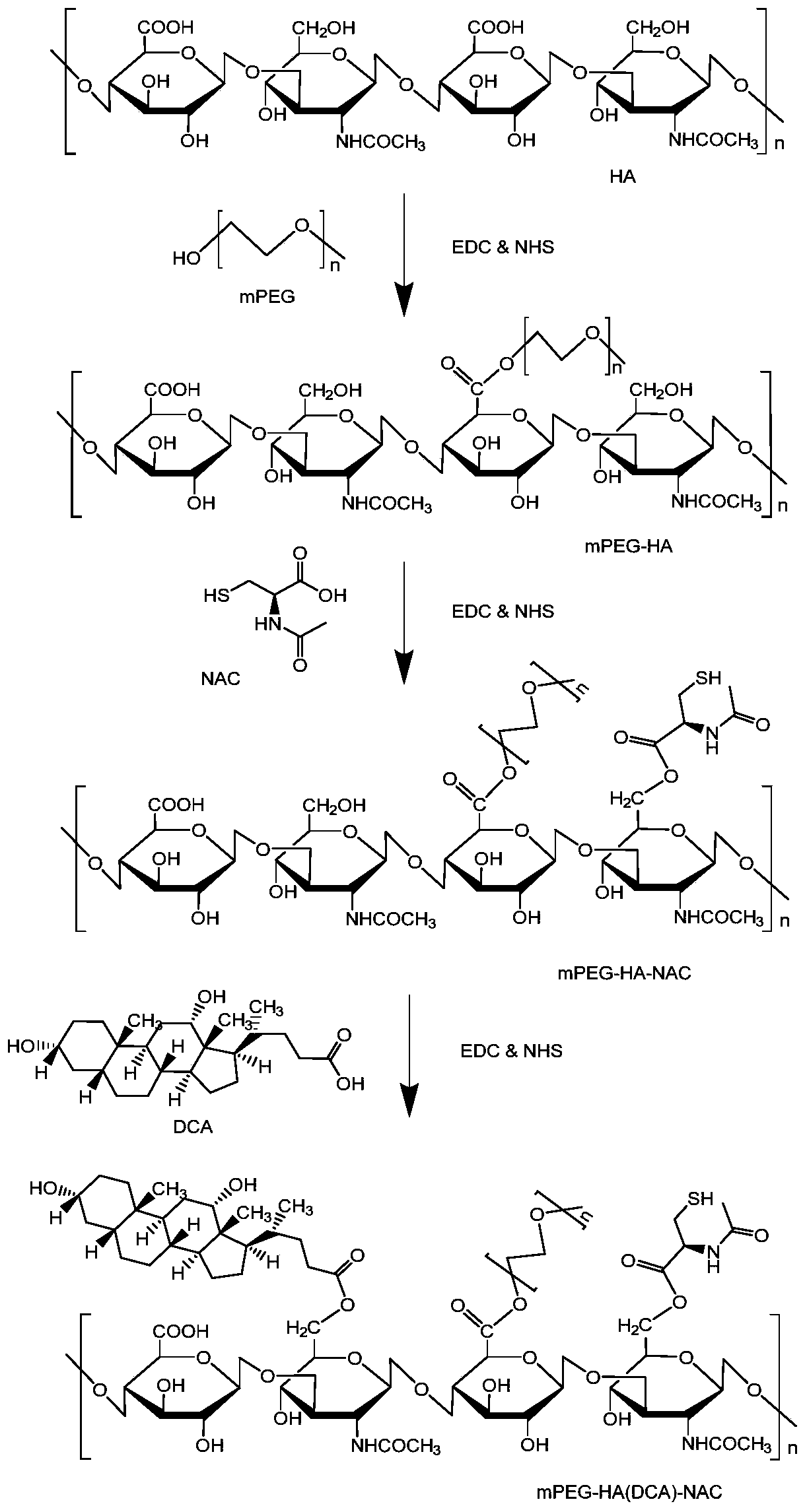
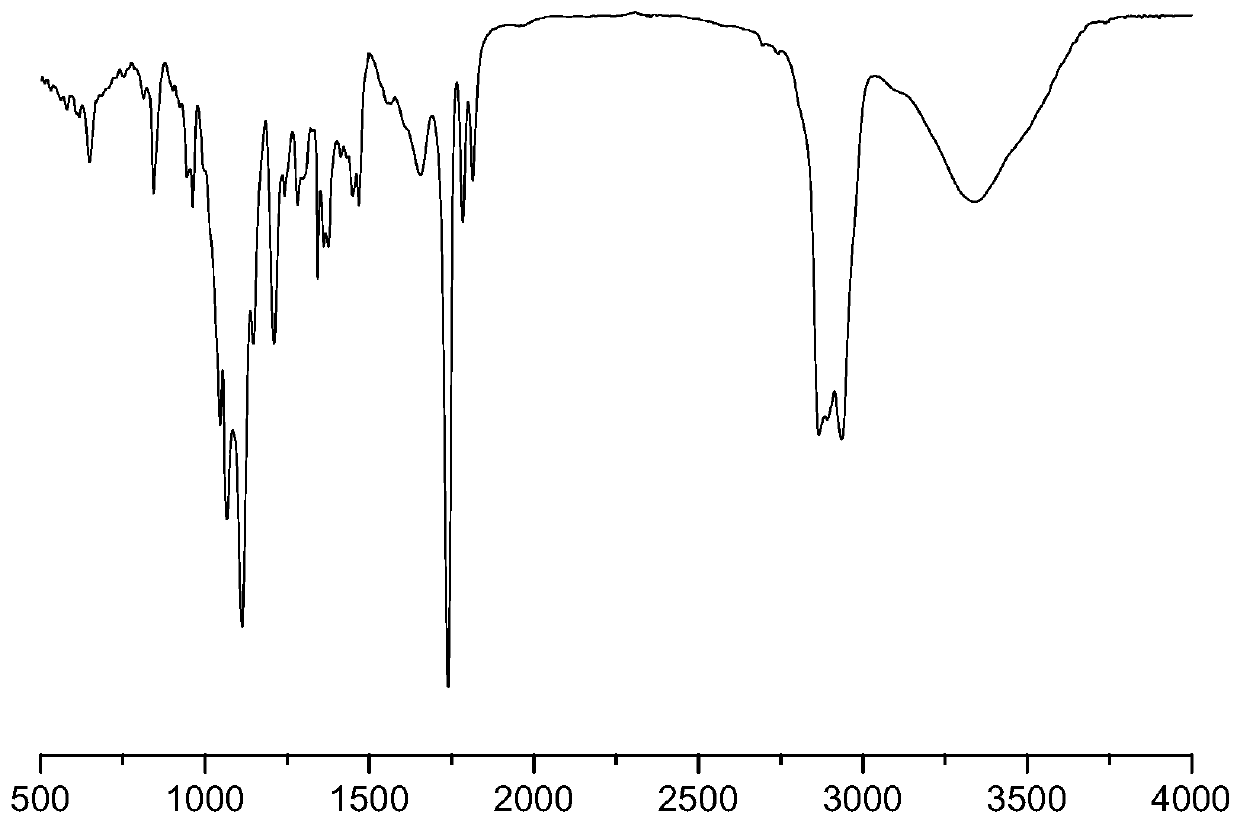
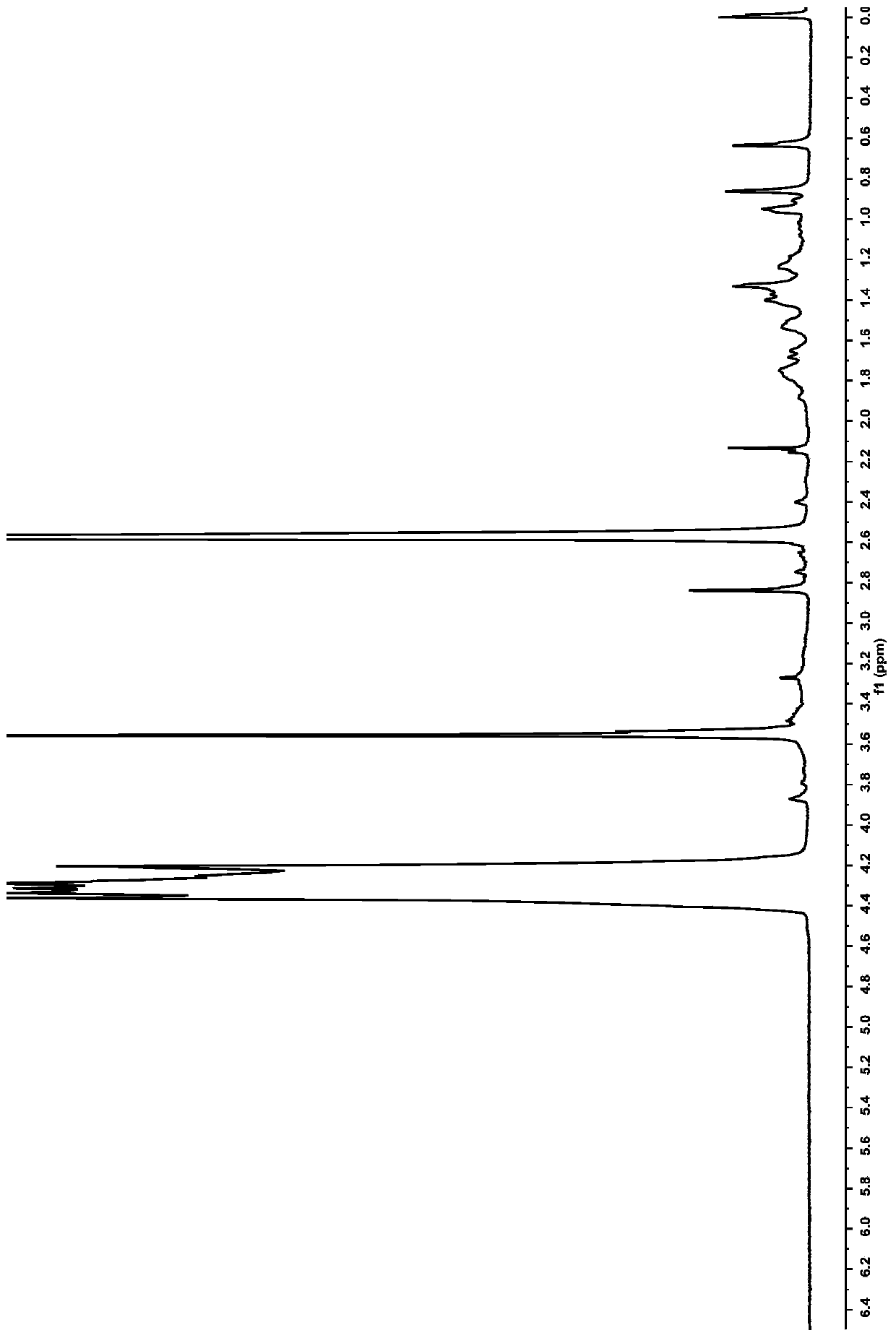
[0063] This embodiment prepares the amphiphilic graft copolymer based on hyaluronic acid, and the preparation method comprises the following steps:
[0064] (1) Synthesis of hyaluronic acid-linked mPEG (abbreviated as HA-mPEG): dissolve hyaluronic acid in the first reaction solvent, add EDC and NHS as the first catalyst, catalyze at 25°C for 2 hours, then add mPEG, The reaction was stirred at 30° C. for 10 hours, dialyzed, centrifuged, and the product was collected and dried to obtain hyaluronic acid-linked mPEG, namely HA-mPEG.
[0065] (2) Synthesis of NAC-DCA-mPEG: N-acetyl-L-cysteine was dissolved in the second reaction solvent, EDC and NHS were used as the second catalyst, and catalyzed at 10° C. for 2 hours; HA-mPEG, stirred and reacted at 35°C for 15 hours, collected the product; the product was dialyzed and freeze-dried to obtain NAC-HA-mPEG.
[0066](3) Synthesis of NAC-HA-mPEG-DCA: Dissolve deoxycholic acid in the third reaction solvent, add EDC and NHS as the thi...
Embodiment 2
[0075] This embodiment prepares the amphiphilic graft copolymer based on hyaluronic acid, and the preparation method comprises the following steps:
[0076] (1) Synthesis of HA-mPEG: Dissolve hyaluronic acid in the first reaction solvent, use DCC as the first catalyst, catalyze at 10°C for 10 hours, then add mPEG, stir and react at 60°C for 3 hours, dialyze for 1 hours, centrifuge, collect the product, and dry to prepare hyaluronic acid-linked mPEG, namely HA-mPEG.
[0077] (2) Synthesis of NAC-HA-mPEG: Dissolve N-acetyl-L-cysteine in the second reaction solvent, use DCC as the second catalyst, and catalyze it at 10°C for 2 hours; then add HA-mPEG , the reaction was stirred at 15°C for 24 hours, and the product was collected; the product was dialyzed for 4 hours and freeze-dried to obtain NAC-HA-mPEG.
[0078] (3) Synthesis of NAC-HA-mPEG-DCA: Dissolve deoxycholic acid in the third reaction solvent, use DCC as the third catalyst, catalyze at 5°C for 1 hour, then add NAC-HA-...
Embodiment 3
[0084] This embodiment prepares the amphiphilic graft copolymer based on hyaluronic acid, and the preparation method comprises the following steps:
[0085] (1) Synthesis of HA-mPEG: Dissolve hyaluronic acid in the first reaction solvent, add DMAP as the first catalyst, catalyze at 55°C for 1 hour, then add mPEG, stir and react at 45°C for 10 hours, dialyze, After centrifugation, the product was collected and dried to obtain HA-mPEG.
[0086] (2) Synthesis of NAC-HA-mPEG: Dissolve N-acetyl-L-cysteine in the second reaction solvent, use DMAP as the second catalyst, and catalyze it at 25°C for 2 hours; then add HA-mPEG , stirred and reacted at 35°C for 15 hours, and collected the product; the product was dialyzed and freeze-dried to obtain NAC-HA-mPEG.
[0087] (3) Synthesis of NAC-HA-mPEG-DCA: Dissolve deoxycholic acid in the third reaction solvent, add EDC and NHS as the third catalyst, catalyze at 35°C for 2 hours, then add NAC-HA- mPEG, stirred at 30°C for 96 hours, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com