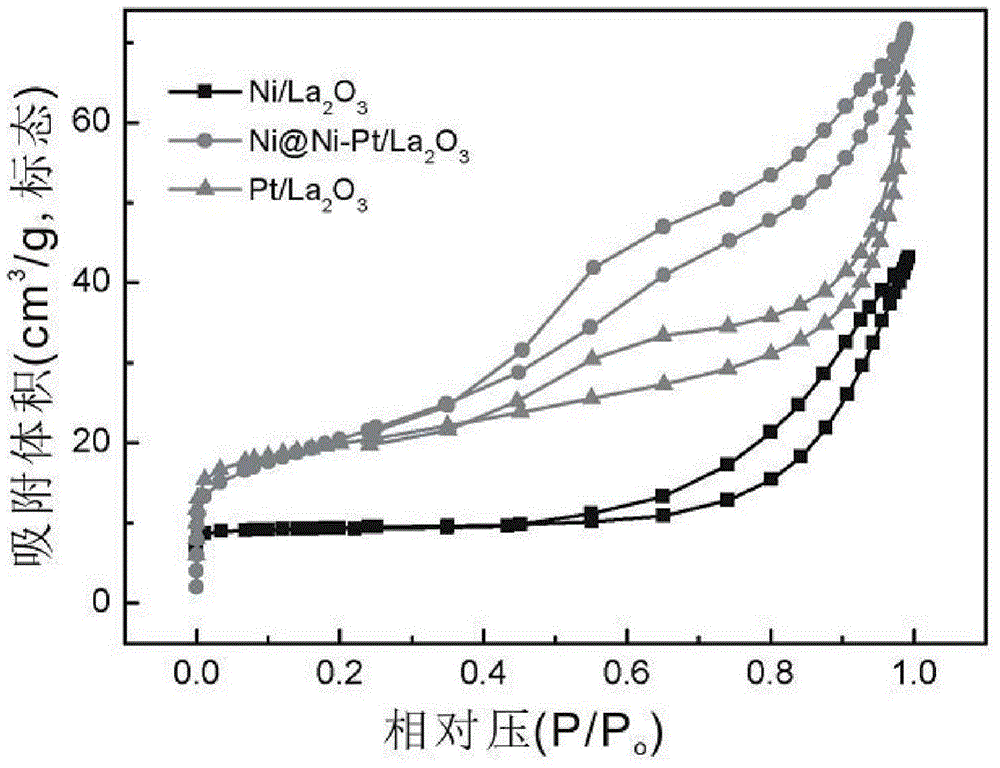
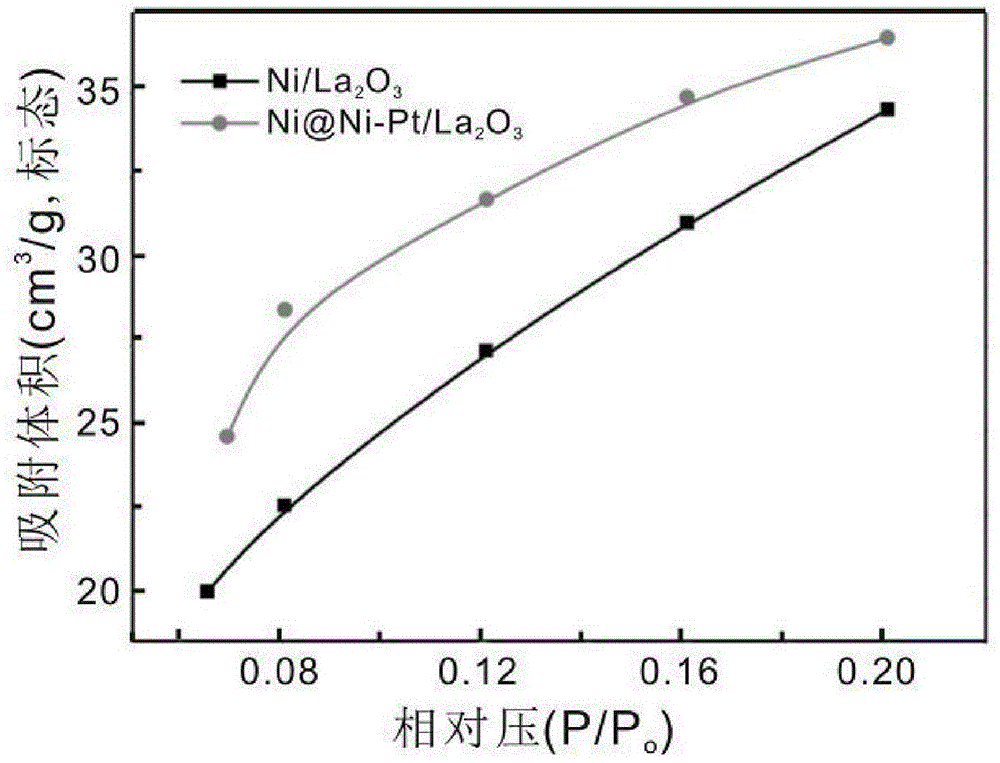
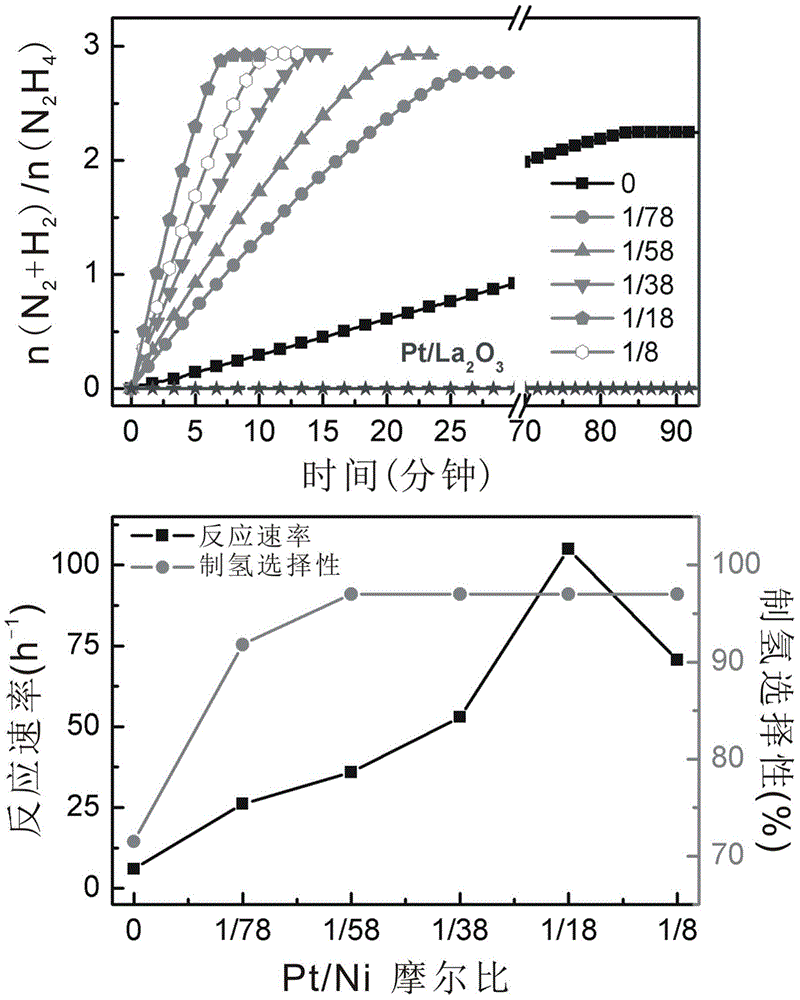
Supported catalyst with kernel-shell structure, preparation method thereof and application
A supported catalyst, shell structure technology, applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of limited practical application, high catalyst preparation cost, high precious metal content, and achieve increased contact , The effect of reducing production cost and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A kind of supported catalyst NiNi-Pt / La with core-shell structure of the present example 2 o 3 , its preparation method is as follows:
[0051] (1) Co-precipitation method to prepare supported non-noble metal precursor Ni / La 2 o 3 :
[0052] 20mL 2.1M TMAH ethanol solution was added dropwise to 60mL 0.083M Ni(NO 3 ) 2 and 0.17M La(NO 3 ) 3 In the ethanol solution, react for 1h, transfer to a reaction kettle lined with tetrafluoroethylene, react for 12h at 100°C and under sealed conditions, obtain a precipitate by centrifugation, dry the precipitate at 60°C for 12h, and then transfer the precipitate into a tube Sintered in an air atmosphere at a temperature of 500 °C for 2 h, and finally in flowing H 2 Atmosphere and 500°C temperature reduction for 1.5h, the supported non-noble metal precursor Ni / La can be obtained 2 o 3 ;
[0053] (2) Preparation of supported catalyst NiNi–Pt / La with core-shell structure by displacement method 2 o 3 :
[0054] Under room t...
Embodiment 2
[0071] A kind of supported catalyst NiNi-Pt / La with core-shell structure of the present example 2 o 3 , its preparation method is as follows:
[0072] (1) Co-precipitation method to prepare supported non-noble metal precursor Ni / La 2 o 3 : same as embodiment 1;
[0073] (2) Preparation of supported catalyst NiNi–Pt / La with core-shell structure by secondary replacement method 2 o 3 :
[0074] Under room temperature and magnetic stirring, the supported non-noble metal precursor Ni / La in step (1) 2 o 3 Place in 20mL K 2 PtCl 6 In aqueous solution (adjust K 2 PtCl 6 The concentration of the aqueous solution is such that the molar ratio of Pt element to Ni element in the catalyst is 1:18) to carry out the first displacement reaction, react for 1h, centrifuge and precipitate, and the precipitate is washed with water and alcohol in turn, and dynamic vacuum dried at 30°C for 12h , and then in the flow H 2 Sintered at 350 °C for 2 h in a tube furnace under atmosphere, and ...
Embodiment 3
[0081] A kind of supported catalyst FeFe-Rh / CeO with core-shell structure of the present example 2 , its preparation method is as follows:
[0082] (1) Co-precipitation method to prepare supported non-noble metal precursor Fe / CeO 2 :
[0083] 20mL 1.6M TMAH ethanol solution was added dropwise to 60mL 0.067M Fe(NO 3 ) 3 and 0.067M Ce(NH 4 ) 2 (NO 3 ) 6 In the ethanol solution, react for 2 hours, transfer to a reaction kettle lined with tetrafluoroethylene, and react for 12 hours at 80°C under sealed conditions. The precipitate is obtained by centrifugation, and the precipitate is dried at 30°C for 12 hours, and then the precipitate is transferred to the tube Sintered in an air atmosphere at 400 °C for 4 h, and finally in flowing H 2 Atmosphere and 450°C temperature reduction for 3h, the supported non-noble metal precursor Fe / CeO can be obtained 2 ;
[0084] (2) Preparation of supported catalyst FeFe–Rh / CeO with core-shell structure by displacement method 2 :
[0085...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com