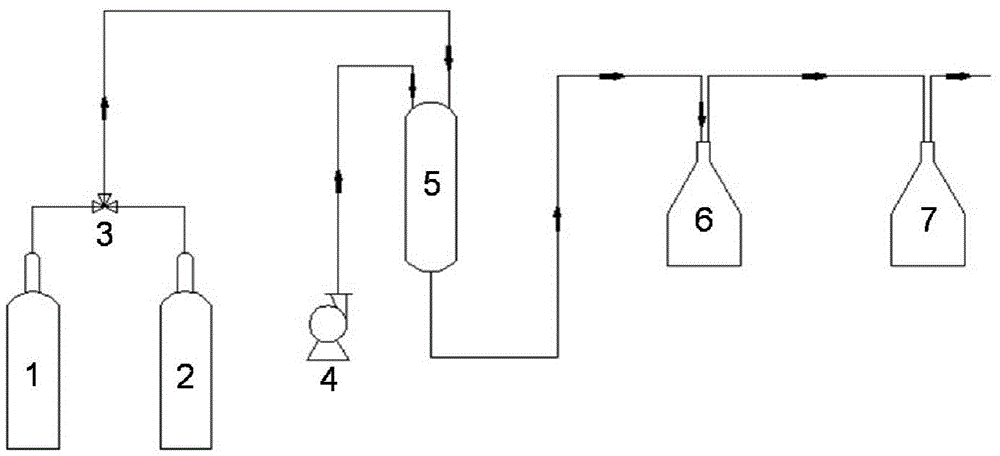
Method for reducing dilute nitric acid to nitrous acid
A technology of dilute nitric acid and nitrous acid, which is applied in the field of coal chemical industry, can solve the problems affecting the ability of blood to transmit oxygen, achieve the prospect of large-scale industrial application, reduce the requirements of equipment, and reduce the effect of separation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The particle size is 20-30 mesh 5g CeO 2 The catalyst was loaded into a glass tube reactor with a diameter of 15mm, and was reduced at 400°C for 5h under a hydrogen atmosphere. Then switch to nitrogen atmosphere, add 1wt% dilute nitric acid reaction solution with a metering pump, the reaction temperature is 40°C, and the WHSV is 1.2h -1 , the reduction reaction was carried out under a nitrogen atmosphere at atmospheric pressure. The conversion rate of nitric acid is 90%, and the selectivity of nitrous acid is 95%.
Embodiment 2
[0019] The particle size is 5g ZrO of 20-30 mesh 2 The catalyst was loaded into a glass tube reactor with a diameter of 15mm, and was reduced at 450°C for 8h under a hydrogen atmosphere. Then switch to a nitrogen atmosphere, add 2wt% dilute nitric acid reaction solution with a metering pump, the reaction temperature is 60°C, and the WHSV is 1.5h -1 , the reduction reaction was carried out under a nitrogen atmosphere at atmospheric pressure. The conversion rate of nitric acid was 88%, and the selectivity of nitrous acid was 97%.
Embodiment 3
[0021] The particle size is 20-30 mesh 5g MoO 3 The catalyst was loaded into a glass tube reactor with a diameter of 15mm, and was reduced at 390°C for 4h under a hydrogen atmosphere. Then switch to nitrogen atmosphere, add 2wt% dilute nitric acid reaction solution with a metering pump, the reaction temperature is 100°C, and the WHSV is 2.0h -1 , the reduction reaction was carried out under a nitrogen atmosphere at atmospheric pressure. The conversion rate of nitric acid is 96%, and the selectivity of nitrous acid is 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com