Cinchona alkaloids compound and preparation method thereof
A kind of technology of cinchonaine and compound, applied in the field of cinchonaline compound and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: the cinchona alkaloid compound of structural formula as shown in formula I:
[0016]
[0017] Wherein, R is 1.
[0018] The preparation method of above-mentioned compound is as follows:
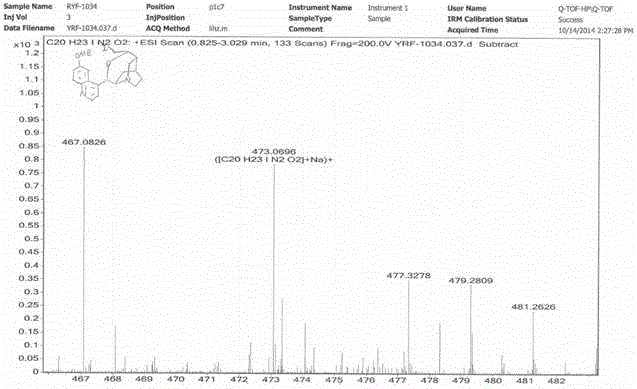
[0019] Weigh 50 mg of quinidine (0.154 mmol, 1 eq) into a 25 mL single-necked bottle, add 10 mL of anhydrous acetonitrile to dissolve, then add 63.8 mg of potassium carbonate (0.46 mmol, 3 eq) to the solution, and place in an ice bath 116.7 mg (0.46 mmol, 3 eq) of iodine was added at the same time, and the mixture was stirred in an ice bath for 3 hours. After the reaction was detected by TLC, the reaction was quenched with aqueous sodium thiosulfite solution, and the aqueous phase was extracted three times with 30 mL of dichloromethane, and the combined organic phase, washed with water and saturated brine successively, dried over anhydrous magnesium sulfate, filtered, evaporated to dryness under reduced pressure, and purified by silica gel column chromatography (eluen...
Embodiment 2
[0020] Embodiment 2: the cinchona alkaloid compound of structural formula as shown in formula I:
[0021]
[0022] Wherein, R is 1.
[0023] The preparation method of above-mentioned compound is as follows:
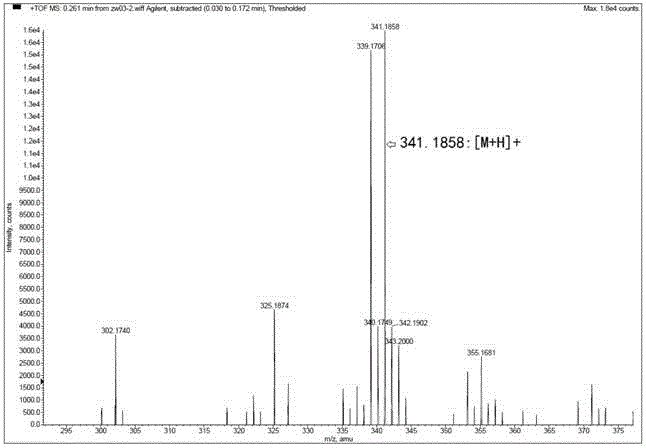
[0024] Weigh 50 mg of quinidine (0.154 mmol, 1 eq) into a 25 mL single-necked bottle, add 10 mL of anhydrous acetonitrile to dissolve, then add 85.7 mg of potassium carbonate (0.62 mmol, 4 eq) to the solution, and place in an ice bath Add 157.4 mg iodine (0.62 mmol, 4eq), and stir the mixture under ice bath for 4 hours. After the reaction is detected by TLC, the reaction is quenched with aqueous sodium thiosulfite solution, and the aqueous phase is extracted 4 times with 30 mL dichloromethane, and the organic phases are combined , washed with water and saturated brine successively, dried over anhydrous magnesium sulfate, filtered, evaporated to dryness under reduced pressure, and purified by silica gel column chromatography (eluent: chloroform / methanol = 20 / 1, v / v) t...
Embodiment 3
[0025] Embodiment 3: the cinchona alkaloid compound of structural formula as shown in formula I:
[0026]
[0027] wherein R is H.
[0028] The preparation method of above-mentioned compound is as follows:
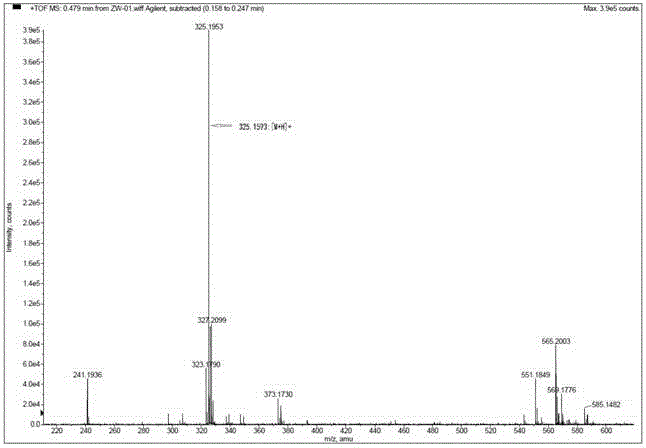
[0029] Weigh 50 mg (0.11 mmol, 1 eq) of the above compound A, place it in a 25 mL single-necked flask, add 10 mL of dry toluene to dissolve, slowly add 62 mg of tributyltin hydride (0.22 mmol, 2 eq) and azobisisobutyronitrile 7 mg (0.044 mmol, 0.4 eq), heated at 120°C for 24 hours under reflux, monitored by TLC, evaporated to dryness under reduced pressure, and purified by silica gel column chromatography (eluent: trichloro Methane / methanol = 4 / 1, v / v), and the light yellow solid was obtained as compound B ( figure 2 ).
[0030] Compound B can be used as the catalyst of N-Nitrosoaldol reaction, and specific examples are as follows:
[0031]
[0032] Add nitrosobenzene (107 mg, 1 mmol) to a 25 mL reaction vial, then add 4 mL of CHCl 3 , stirred well until disso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com