Method for preparing 2,6-dichlorotoluene employing Hbeta molecular sieve and catalytic o-chlorotoluene
A technology for catalyzing ortho- and dichlorotoluene by molecular sieve is applied in chemical instruments and methods, preparation of halogenated hydrocarbons, organic chemistry, etc., and can solve the problems of difficult to realize industrialization, slow chlorination reaction speed, complicated reaction conditions, etc., and achieve easy product The effect of separation, simple operation and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Preparation of Hβ molecular sieve: 5 g β molecular sieve and 50 mL NH 4 NO 3 The solution was added into a 100 mL three-necked flask, stirred at 90 °C for 1 h, filtered and washed, and the obtained sample was dried in an oven at 120 °C for 2 h. Calcined at 500 °C for 2 h in a muffle furnace to obtain Hβ molecular sieves.
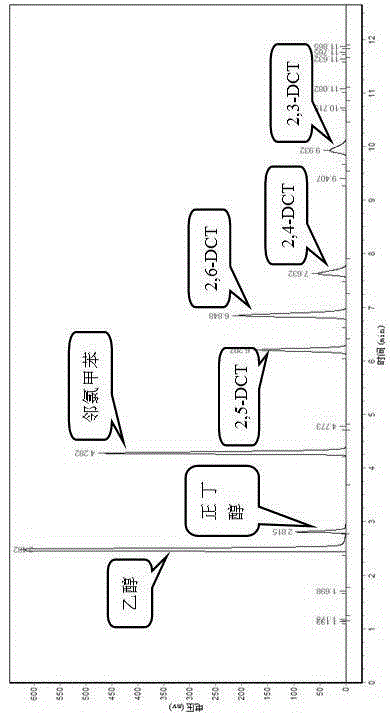
[0028] (2) Chlorination reaction step of o-chlorotoluene Add 141 mL (150 g) of o-chlorotoluene into a 250 mL four-neck flask, add 3.0 g of Hβ molecular sieve catalyst, stir to disperse evenly; then pass 50 mL / Min chlorine gas dried with concentrated sulfuric acid, reacted at 35 °C for 12 h to obtain dichlorotoluene, after the tail gas was condensed, it was absorbed by NaOH solution to remove unreacted Cl 2 .
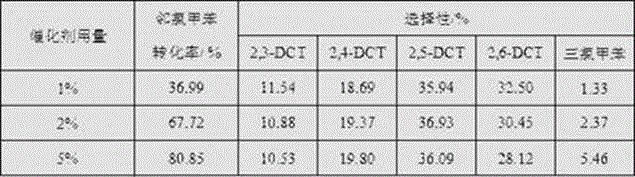
[0029] Change the amount of Hβ molecular sieve to 1.5 g, 7.5 g, the influence of the amount of Hβ molecular sieve catalyst on the chlorination process of o-chlorotoluene can be obtained as shown in Table 1:
[0030] Table 1 Effe...
Embodiment 2
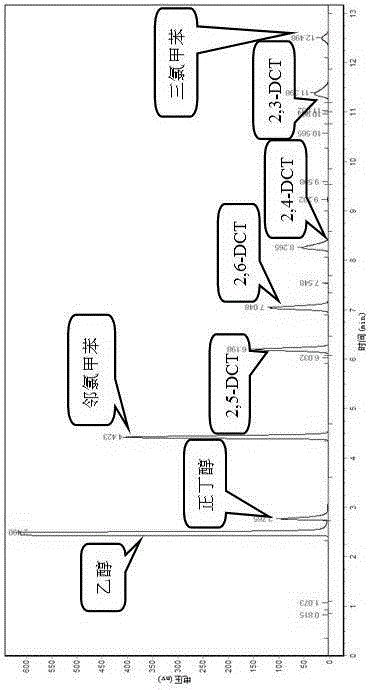
[0036] The same method as in Example 1 is used to prepare the Hβ molecular sieve catalyst. The chlorination process of o-chlorotoluene adopts the same method as in Example 1, but the reaction time is changed to 3 h, 6 h, and 9 h to obtain different reaction times for o-chlorotoluene chloride. The impact of the chemical process, as shown in Table 2:
[0037] Table 2 Effects of different reaction times on the chlorination of o-chlorotoluene
[0038]
[0039] Table 2 reflects the impact of reaction time on the chlorination of o-chlorotoluene; as can be seen from the table, as the reaction time is extended from 3 h to 12 h, the conversion rate of o-chlorotoluene increases from 10.36% to 67.72%; 2 , the selectivity of 6-DCT gradually decreased from 34.36% to 30.45%.
[0040] The experimental results show that prolonging the reaction time is conducive to the improvement of the conversion rate of o-chlorotoluene and the increase of the selectivity of by-products, which will l...
Embodiment 3
[0042] Adopt the same method of embodiment 1 to prepare Hβ molecular sieve catalyst, adopt the same method of embodiment 1 in the o-chlorotoluene chlorination process, but change reaction temperature to be 50 ℃, 65 ℃, can obtain different reaction temperatures to the o-chlorotoluene chlorination process Impact, as shown in Table 3:
[0043] Table 3 Effects of different reaction temperatures on the chlorination of o-chlorotoluene
[0044]
[0045] Table 3 reflects the impact of reaction temperature on the chlorination of o-chlorotoluene; as can be seen from Table 3, as the reaction temperature increases from 35 °C to 65 °C, the conversion of o-chlorotoluene increases from 67.72% to 90.15%; The selectivity of 2,6-DCT decreased from 30.45% to 26.81%; this indicates that low temperature is beneficial to the increase of the selectivity of 2,6-DCT, but it also inhibits the conversion rate of o-chlorotoluene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com