Folded chlorotoxin, chlorotoxin mutant, folded chlorotoxin mutant and preparation process thereof
A chlorinated toxin and a preparation process technology, applied in the field of folded chlorinated toxins, can solve the problems of lack of research and innovation of folded structure and variant structure, limited application potential of chlorinated toxins, etc., achieve good economic and social benefits, and realize automatic control , the effect of simple separation and purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
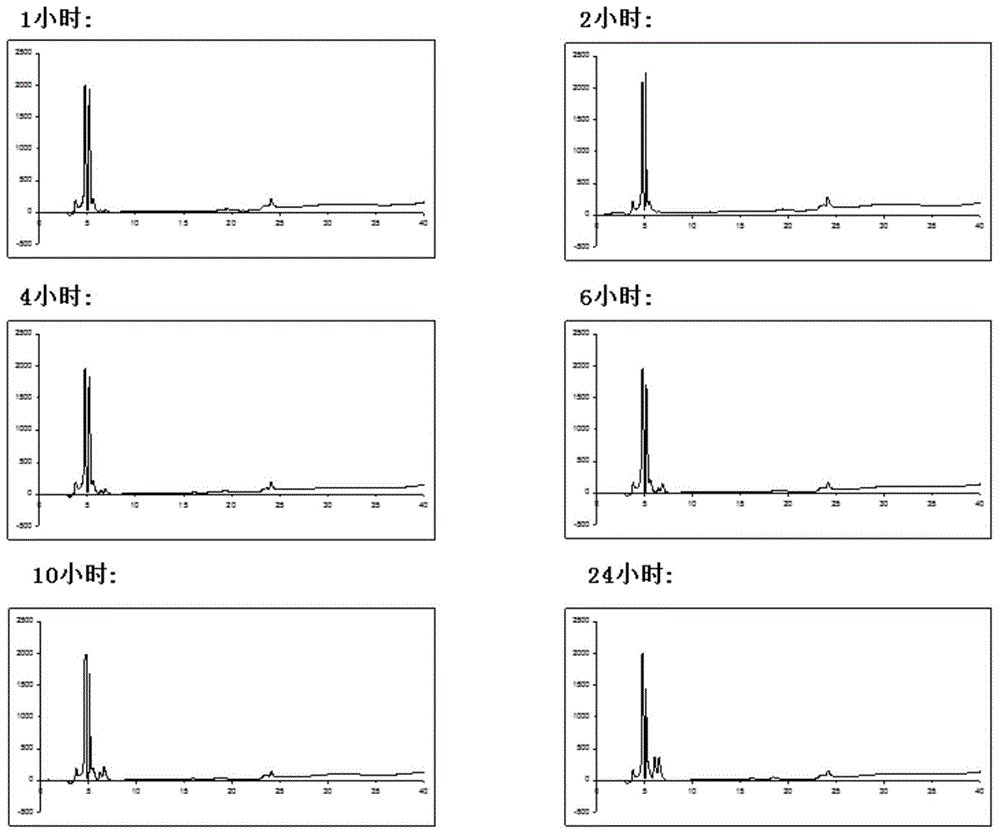
[0045] Embodiment 1: Chlorotoxin (40 mg) is weighed and placed in a test tube containing 100 mmol of ammonium bicarbonate, 2 mmol of guanidine hydrochloride, 10 mmol of glutathione, and 10% dimethyl sulfoxide mixed solution , vortex at least 3 times until the solution is clear and clear. The system was placed in a refrigerator at 4 degrees Celsius, and the product was taken out at different time points (1 hour, 2 hours, 4 hours, 6 hours, 10 hours, 24 hours). High performance liquid chromatography uses Dionex C18 Acclaim 120 analytical column as the chromatographic column, the flow rate is 1.00 ml / min, and the elution phase is: 0.1% trifluoroacetic acid aqueous solution; 0.1% trifluoroacetic acid acetonitrile solution, 5-65% gradient elution 30 Minutes, get the separation spectrum of the folded chlorinated toxin.
Embodiment 2
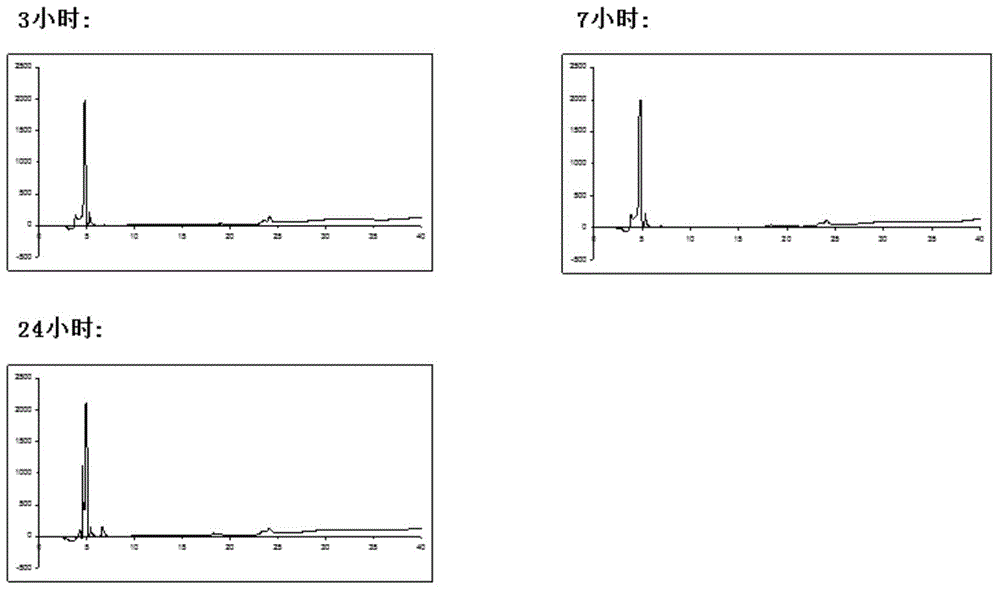
[0046] Embodiment 2: Place chlorinated toxin (40 milligrams) in the test tube that 100 millimoles ammonium bicarbonate, 4 millimoles guanidine hydrochloride, 10 millimoles glutathione, 10% dimethyl sulfoxide mixed solution are placed after weighing , vortex at least 3 times until the solution is clear and clear. The system was placed in a refrigerator at 4 degrees Celsius, and the product was taken out at different time points (3 hours, 7 hours, 24 hours). High performance liquid chromatography uses Dionex C18 Acclaim 120 analytical column as the chromatographic column, the flow rate is 1.00 ml / min, and the elution phase is: 0.1% trifluoroacetic acid aqueous solution; 0.1% trifluoroacetic acid acetonitrile solution, 5-65% gradient elution 30 Minutes, get the separation spectrum of the folded chlorinated toxin.
Embodiment 3
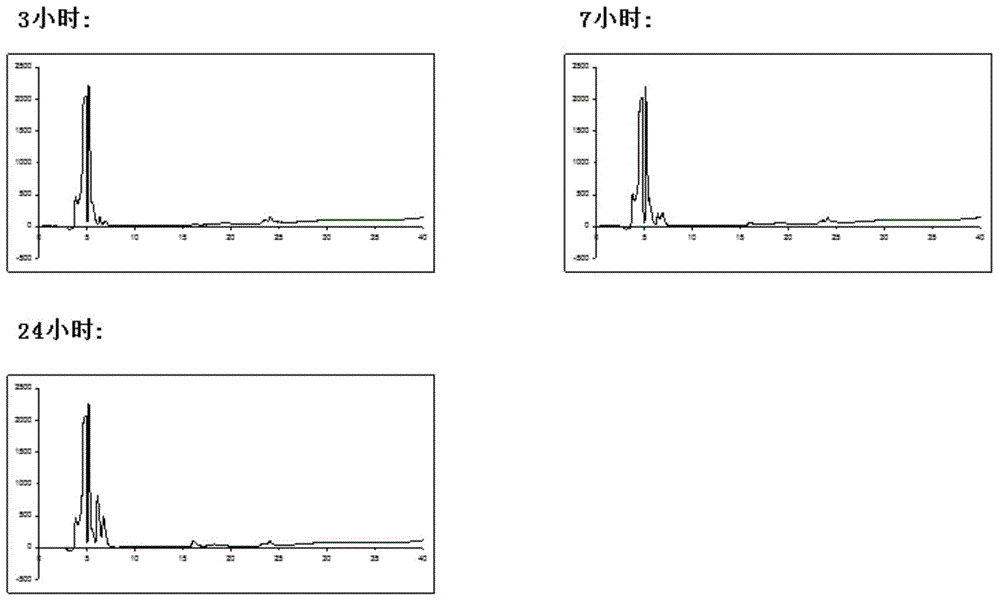
[0047] Embodiment 3: after weighing chlorinated toxin (40 milligrams), be placed in the test tube that 100 millimoles ammonium bicarbonate, 2 millimoles guanidine hydrochloride, 100 millimoles glutathione, 10% dimethyl sulfoxide mixed solution are placed , vortex at least 3 times until the solution is clear and clear. The system was placed in a refrigerator at 4 degrees Celsius, and the product was taken out at different time points (3 hours, 7 hours, 24 hours). High performance liquid chromatography uses Dionex C18 Acclaim 120 analytical column as the chromatographic column, the flow rate is 1.00 ml / min, and the elution phase is: 0.1% trifluoroacetic acid aqueous solution; 0.1% trifluoroacetic acid acetonitrile solution, 5-65% gradient elution 30 Minutes, get the separation spectrum of the folded chlorinated toxin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com