Actinoalloteichus sp., three antifungal maclafungin compounds, and preparation method and application of compounds
A heterogeneous actinomycetes and compound technology, applied in the field of microorganisms and new pharmaceuticals and pesticides, can solve the problems that the antibacterial mechanism and biosynthesis mechanism have not been reported, and achieve good application prospects, strong antibacterial and insecticidal effects, and high purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The identification of embodiment 1 deep-sea actinomycetes SHA6
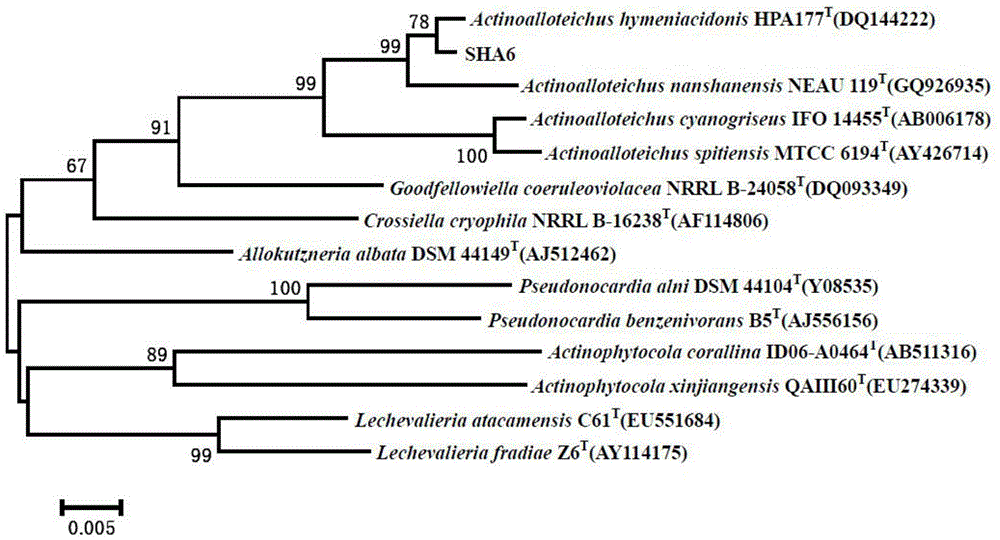
[0033] Actinomycetes SHA6, isolated from -3587m deep-sea sediments in the South China Sea (17°59'48.135N, 114°34'16.668E), was preserved in the China Center for Type Culture Collection on February 20, 2014, and the preservation number is: CCTCC NO: M2014041, taxonomically named Actinoalloteichus sp. SHA6, and the depository address is China. Wuhan. Wuhan University.
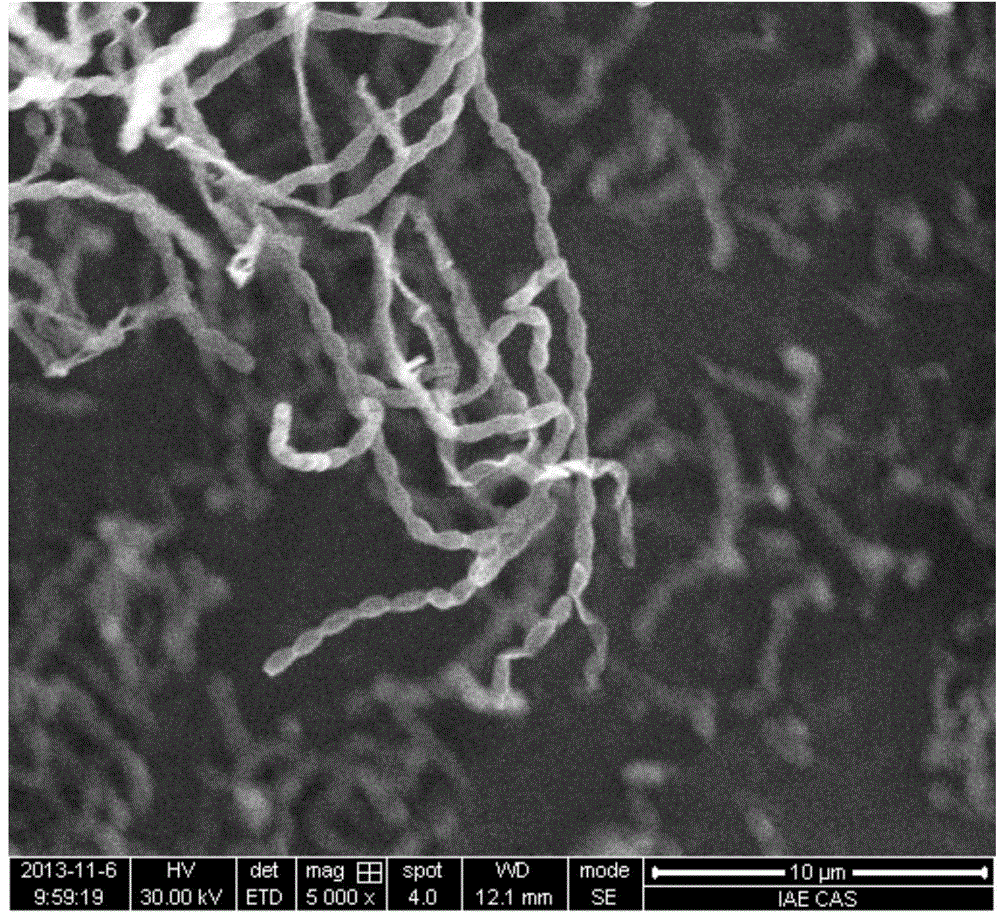
[0034] Strain SHA6 cells are Gram-positive, forming straight and long sporocydia with a spore size of about 1.0×0.7 μm; grow well at 28-37°C, grow slowly at 4°C and 8°C; grow on PDA and modified Emerson's medium well and form spore filaments ( figure 1), melanin is often produced when cultured for a long time; it can tolerate 12% NaCl concentration, and the optimum growth NaCl concentration is 0-5%; it can tolerate the pH value of 6-11.6, and the optimum growth pH is 7-11; It can grow under both oxygen and microaerophilic conditions; it can...
Embodiment 2
[0038] Example 2 Fermentation of strain SHA6 to prepare maclafungin compounds
[0039] 2.1 Strain activation: inoculate the strain SHA6 on the prepared ISP3 medium plate by streaking method, and culture at a constant temperature of 28°C for 5 days;
[0040] The ISP3 medium is as follows: boil 20.0g of oatmeal with 1L of water for about 30min, filter through four layers of gauze to obtain the filtrate, and make up to 1L with water, 1mL of trace element mother solution, 20g of agar, 1L of distilled water, pH7.2. Trace element mother solution (1000×): MgSO 4 ·7H 2 O1.0g, ZnSO 4 ·7H 2 O1.0g, MnCl 2 4H 2 O1.0g, FeSO 4 ·7H 2 O1.0g, CoCl 2 ·6H 2 O1.0g, CaCl 2 1.0g, H 3 BO 3 1.0g.
[0041] 2.2 Preparation of seed solution: Use a puncher to take a 5mm diameter bacterial block on the plate where the strain grows densely, inoculate it in a 250mL triangular flask filled with 50mL PSB medium containing artificial sea salt, and culture it on a constant temperature shaker at 28°...
Embodiment 3
[0050] The structural analysis of embodiment 3 Maclafungin compounds
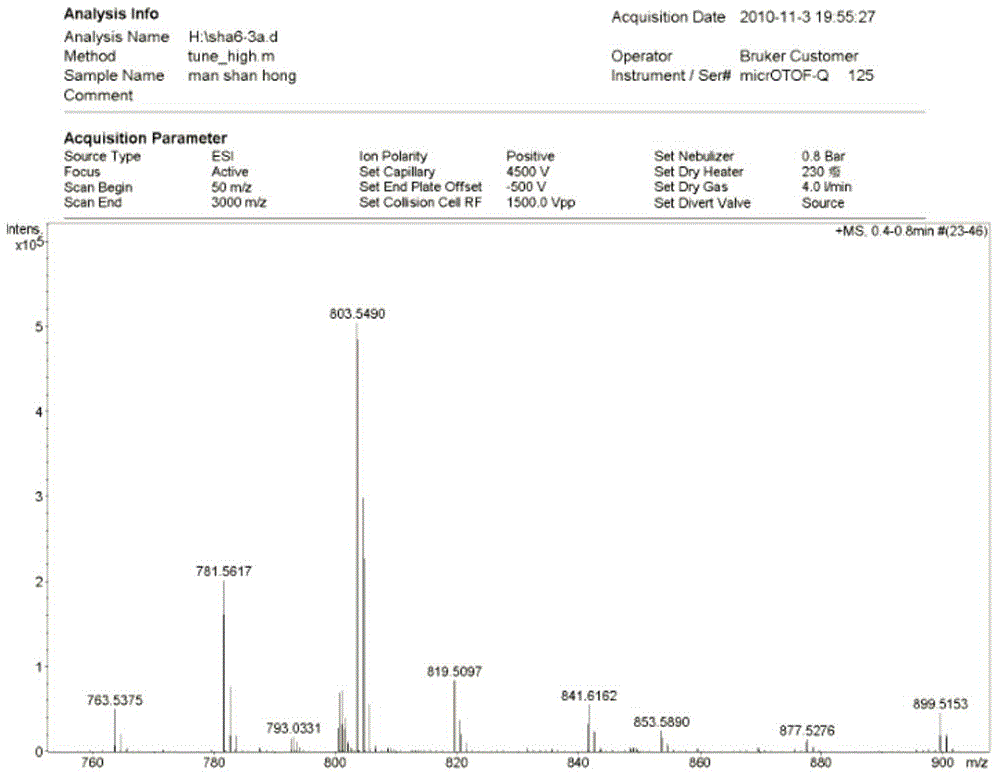
[0051] Maclafungin B (3a): white powder, HR-ESI-MS showed ( image 3 ): m / z781.5617[M+H] + (Calcd. for C 44 h 77 o 11 , 781.5466) and m / z803.5490[M+Na] + (Calcd. for C 44 h 76 o 11 Na, 803.5285), deduced that its molecular formula is C 44 h 76 o 11 , whose degree of unsaturation is 7.
[0052] Compound 3a 1 H NMR (600MHz, CDCl 3 ) (Table 1) 6 methyl signals are given in the spectrum δ H 1.22 (3H, d, J=6.1Hz), 1.14 (3H, d, J=6.4Hz), 0.90 (3H, d, J=7.1Hz), 0.85 (3H, d, J=6.9Hz), 0.80 ( 3H, t, J=7.4Hz), 0.74 (3H, d, J=6.8Hz); 1 methoxy signal δ H 3.35 (3H, s); 3 pairs of trans double bond signal δ H6.57 (1H, dd, J=15.6, 10.2Hz), 7.77 (1H, d, J=15.6Hz), 6.08 (1H, dd, J=14.3, 10.7Hz), 5.41 (1H, m), 5.92 (1H , dd, J=14.8, 10.7Hz), 5.33 (1H, dd, J=14.8, 9.8Hz); proton signals on multiple oxygenated carbons (the signals overlap a lot), such as δ H 4.05, 4.03, 3.79, 3.74, 3.64, 3.54, 3.42, etc. 13...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com