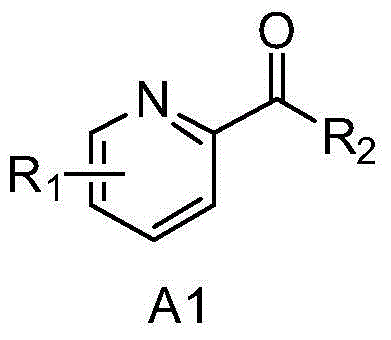
Asymmetric catalytic hydrogenation method for 2-pyridinone compounds
An asymmetric and compound technology, applied in the direction of organic chemistry, etc., to achieve good industrialization prospects, good catalytic effect, and high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
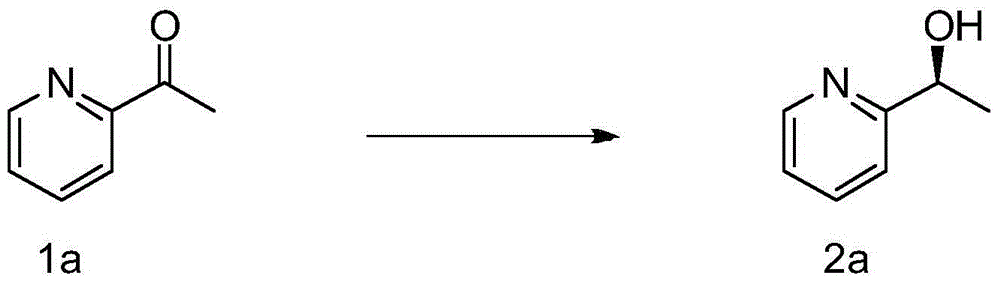
[0026] Example 1: Preparation of (2a) by hydrogenation of (1a)
[0027]
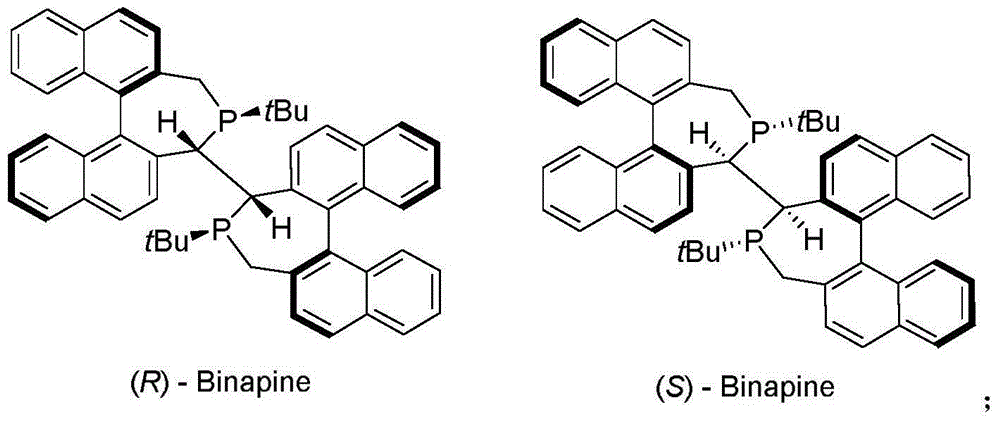
[0028] In a glove box, rhodium bis(1,5-cyclooctadiene)tetrafluoroborate (0.41 mg, 0.001 mmol, 1 mol%) and the chiral ligand (R)-Binapine, 0.0011 mmol, 0.81 mg, 1.1 mol %) was dissolved in dichloromethane (1 mL), stirred at 25° C. for 1 hour, and compound 1a (0.1 mmol) and dichloromethane (1 mL) were added. The reaction system was placed in an autoclave at 25 °C and H 2 (10atm) under the condition of stirring and reacting for 20 hours. Then the solvent was removed under reduced pressure, separated by column chromatography (taking silica gel column, eluent: ethyl acetate / petroleum ether=1 / 2), to obtain pure product 2a, the product was a colorless liquid, and the conversion rate was 99%. HPLC analysis, measured ee value (ee=99%). 1 H NMR (400MHz, CDCl 3 )δ8.53(d,J=4.8Hz,1H),7.70(td,J=8.0,2.0Hz,1H),7.30(d,J=7.6Hz,1H),7.19–7.22(m,1H), 4.90(dd,J=12.8,6.4Hz,1H),4.53(s,1H),1.51(d,J=6.8Hz,3H); 13 C NMR (...
Embodiment 2
[0030] Example 2: Preparation of (2b) by hydrogenation of (1b)
[0031]
[0032] In a glove box, rhodium bis(1,5-cyclooctadiene)tetrafluoroborate (0.41 mg, 0.001 mmol, 1 mol%) and the chiral ligand (R)-Binapine, 0.0011 mmol, 0.81 mg, 1.1 mol %) was dissolved in dichloromethane (1 mL), stirred at 25° C. for 1 hour, and compound 1b (0.1 mmol) and dichloromethane (1 mL) were added. The reaction system was placed in an autoclave at 25 °C and H 2 (10atm) under the condition of stirring and reacting for 20 hours. Then the solvent was removed under reduced pressure, separated by column chromatography (taking silica gel column, eluent: ethyl acetate / petroleum ether=1 / 2), to obtain pure product 2b, the product was analyzed by NMR, and the conversion rate was 99%, and it was analyzed by HPLC Analysis, measured ee value (ee=91%). 1 H NMR (400MHz, CDCl 3 )δ8.51(d, J=4.8Hz, 1H), 7.67(td, J=7.6, 1.2Hz, 1H), 7.28(d, J=8.0Hz, 1.0H), 7.16-7.19(m, 1H) ,4.68(dd,J=6.4,5.2Hz,1H),4.48(s,1H)...
Embodiment 3
[0033] Example 3: Preparation of (2c) by hydrogenation of (1c)
[0034]
[0035] In a glove box, rhodium bis(1,5-cyclooctadiene)tetrafluoroborate (0.41 mg, 0.001 mmol, 1 mol%) and the chiral ligand (R)-Binapine, 0.0011 mmol, 0.81 mg, 1.1 mol %) was dissolved in dichloromethane (1 mL), stirred at 25° C. for 1 hour, and compound 1c (0.1 mmol) and dichloromethane (1 mL) were added. The reaction system was placed in an autoclave at 25 °C and H 2 (10atm) under the condition of stirring and reacting for 20 hours. Then the solvent was removed under reduced pressure, separated by column chromatography (taking silica gel column, eluent: ethyl acetate / petroleum ether=1 / 2), to obtain pure product 2c, the product was analyzed by NMR, and the conversion rate was 99%, and it was analyzed by HPLC Analysis, measured ee value (ee=84%).1 H NMR (400MHz, CDCl 3 )δ8.57(d, J=4.8Hz, 1H), 7.70(td, J=7.6, 1.6Hz, 1H), 7.20-7.27(m, 2H), 4.58(d, J=4.4Hz, 1H), 4.26(s,1H),2.01-2.09(m,1H),1.04(d,J=7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com