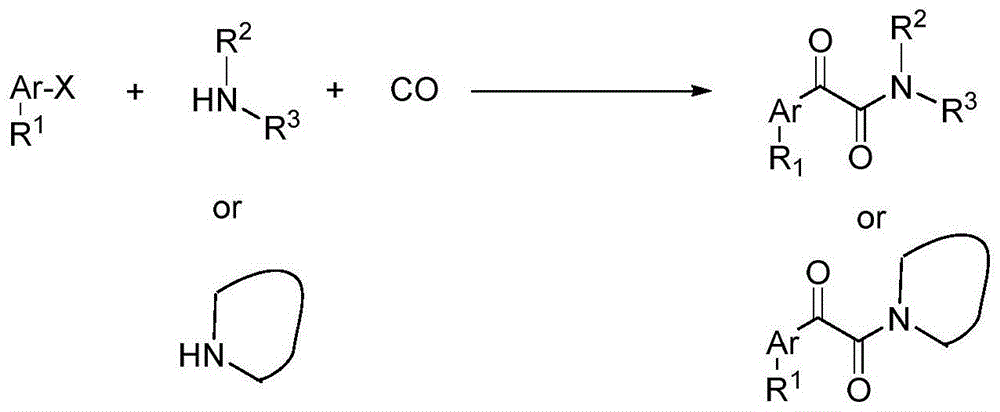
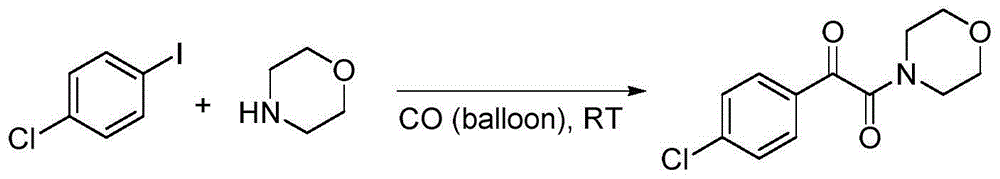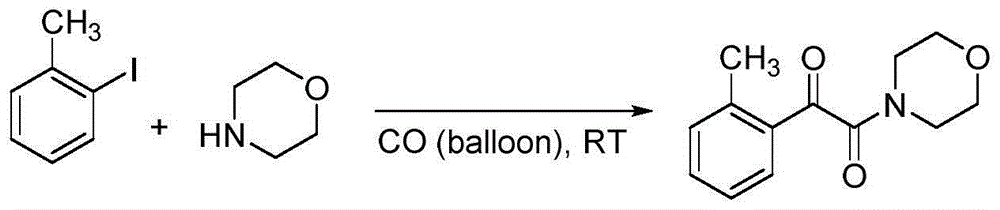Catalytic carbonylation method for synthesis of alpha-keto amide
A technology of ketamides and compounds, which is applied in the field of catalytic carbonylation to synthesize α-ketoamides, can solve the problems of insufficient application range, increased production costs, and strict reaction conditions, and achieve easy operation, stable reaction raw materials, and selectivity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029]Add palladium acetate (0.01mmol), 1-chloro-4-iodobenzene (0.5mmol), morpholine (0.5mmol), sodium carbonate (1.0mmol) and polyethylene glycol-400 (2.0g) to the 25mL reaction flask in sequence , and introduce one atmosphere of carbon monoxide. The mixture was stirred and reacted at room temperature for 6 hours, 15 mL of saturated brine was added, and extracted three times with ethyl acetate. Finally, the organic solvent was distilled off under reduced pressure and separated by column chromatography to obtain a white solid product with a yield of 80%.
[0030] 1 H NMR (400MHz, CDCl 3 ): δ7.89(d, J=8.0Hz, 2H), 7.48(d, J=8Hz, 2H), 3.79-3.73(m, 4H), 3.64(t, J=4Hz, 2H), 3.36ppm ( t, J = 4Hz, 2H); 13 C NMR (100MHz, CDCl 3 ): δ189.7, 164.9, 141.6, 131.5, 131.1, 129.5, 66.7, 66.6, 46.3, 41.7ppm; mp: 116.0-116.5°C.
Embodiment 2
[0032]
[0033] Add palladium acetate (0.01mmol), 1-methyl-2-iodobenzene (0.5mmol), morpholine (0.5mmol), sodium carbonate (1.0mmol) and polyethylene glycol-400 (2.0g ), and introduce one atmosphere of carbon monoxide. The mixture was stirred and reacted at room temperature for 9 hours, 15 mL of saturated brine was added, and extracted three times with ethyl acetate. Finally, the organic solvent was distilled off under reduced pressure and separated by column chromatography to obtain a yellow solid product with a yield of 93%.
[0034] 1 H NMR (400MHz, CDCl 3 ): δ7.70(d, J=8Hz, 1H), 7.47(td, J=8, 1.2Hz, 1H), 7.33-7.28(m, 2H), 3.78-3.74(m, 4H), 3.65(t , J=8Hz, 2H), 3.37(t, J=8Hz, 2H), 2.64ppm(s, 3H); 13 C NMR (100MHz, CDCl 3 ): δ193.1, 166.2, 141.6, 133.9, 132.7, 132.7, 131.5, 126.2, 66.7, 66.6, 46.3, 41.6, 21.8ppm.
Embodiment 3
[0036]
[0037] Add palladium acetate (0.01mmol), 1,3-dimethyl-5-iodobenzene (0.5mmol), morpholine (0.5mmol), sodium carbonate (1.0mmol) and polyethylene glycol-400 successively into 25mL reaction flask (2.0g), and introduce one atmosphere of carbon monoxide. The mixture was stirred and reacted at room temperature for 9 hours, 15 mL of saturated brine was added, and extracted three times with ethyl acetate. Finally, the organic solvent was distilled off under reduced pressure and separated by column chromatography to obtain a white solid product with a yield of 84%.
[0038] 1 H NMR (400MHz, CDCl 3 ): δ7.53(s, 2H), 7.26(s, 1H), 3.79-3.74(m, 4H), 3.63(t, J=4.8Hz, 2H), 3.34(t, J=4.8Hz, 2H) , 2.35ppm (d, J=0.5Hz, 6H). 13 C NMR (100MHz, CDCl 3 ): δ191.6, 165.7, 138.9, 136.8, 133.1, 127.3, 66.7, 66.7, 46.2, 41.6, 21.1ppm. HRMS (ESI) calcd for C 14 h 17 NO 3 [M+Na] m / z 270.110065, found m / z 270.111397; IR: ν max (KBr)=3458, 2965, 2921, 2893, 2850, 1755, 1675, 1653, 1592,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com