Amphiphilic polymer as well as preparation method and application thereof
A technology of amphiphilic polymers and polymers, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as strategies and limitations that have not been developed in modularization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] The invention also provides a method for preparing the amphiphilic polymer, including:
[0066] A preparation method of amphiphilic polymer, including:
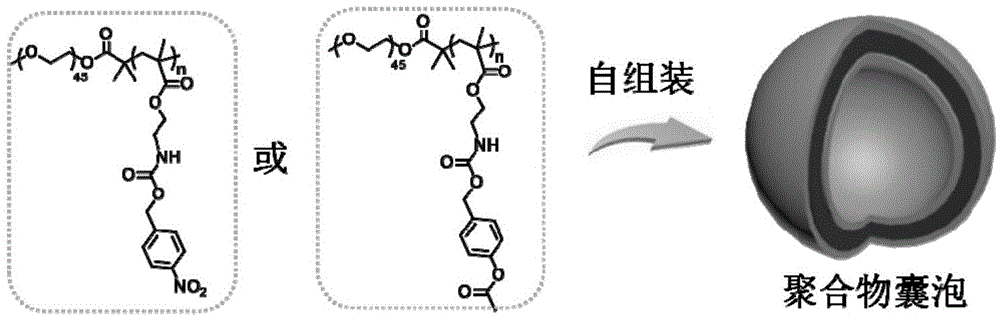
[0067] Polymerizing a polymer having a structure of formula (II) with a monomer having a structure of formula (III) to obtain a polymer having a structure of formula (I);
[0068]
[0069] Among them, M 1 For formula (101), formula (102), formula (103), formula (104), formula (105), formula (106), formula (107), formula (108), formula (109), formula (110) And one or more of (111),
[0070]
[0071] Among them, p is from 11 to 445, and q is from 10 to 200;
[0072]
[0073] Among them, m is 1-11;
[0074] M 2 Is formula (202), formula (203) or formula (204),
[0075] Among them, x is 0-19 and y is 0-19;
[0076]
[0077] According to the present invention, the present invention polymerizes the polymer having the structure of formula (II) with the monomer having the structure of formula (III) to obtain the polymer of the structure ...
Embodiment 1
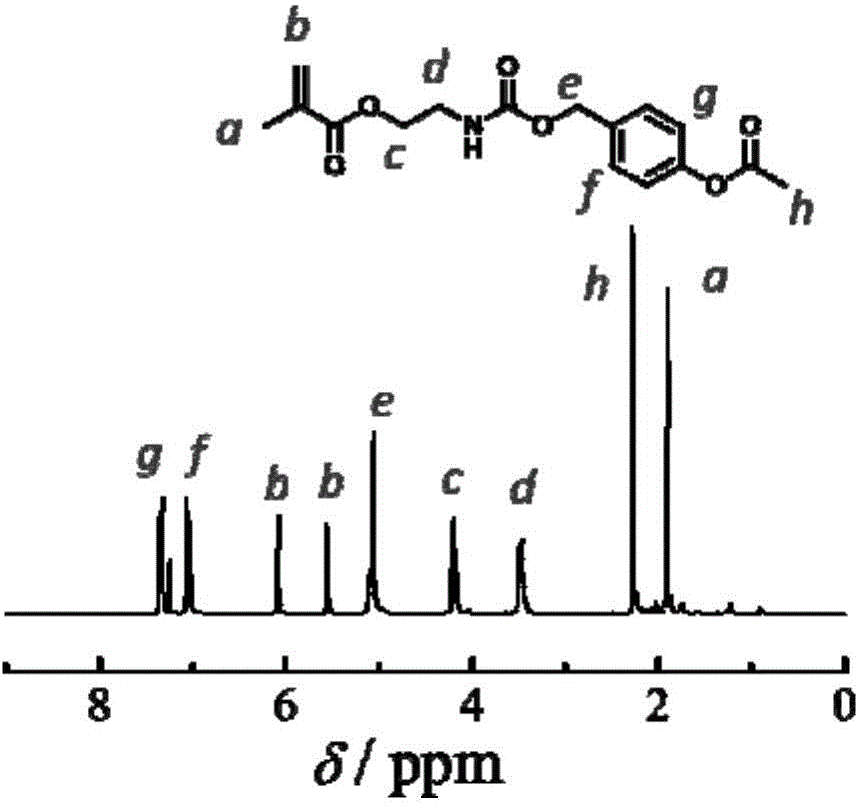
[0095] 1) Preparation of a compound with a structure represented by the hydrophobic monomer formula (III-1) (where m=1, x=0):
[0096]
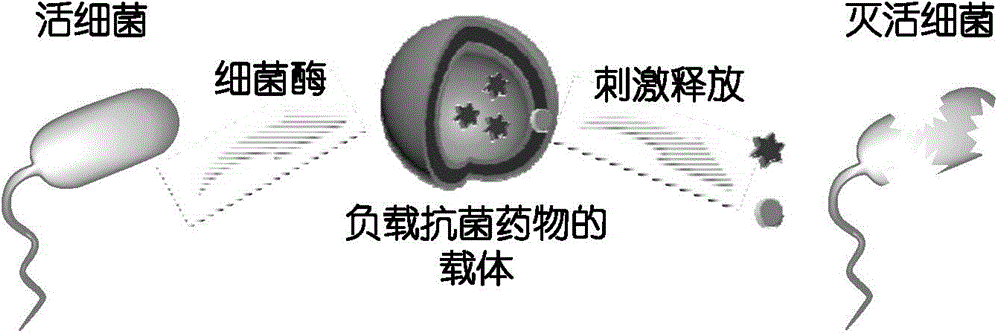
[0097] Its characteristic is: bacterial esterase can cut off the acetyl group of the end group, and then the 1,6-electron rearrangement of p-hydroxybenzyl alcohol will remove a molecule of carbon dioxide and finally release a primary amine group.
[0098] Preparation method: Dissolve p-hydroxybenzyl alcohol (5.0g, 40mmol) and triethylamine (4.45g, 44mmol) in 120mL of dry dichloromethane, slowly add acetyl chloride (3.14g) while cooling in an ice bath , 40mmol). Stir overnight, filter with suction, concentrate and purify by column to obtain 2.3 g of 4-hydroxymethyl acetophenone ester with a yield of 35%.
[0099] Dissolve 4-hydroxymethyl acetophenate (2.3g, 13.8mmol) and a catalytic amount of dibutyltin dilaurate (50μL) in 80mL of dry dichloromethane. Add methyl slowly while stirring at room temperature. Isocyanoethyl acrylate (3.2 g, 20.8 mmol). ...
Embodiment 2
[0128] 1) Preparation of a compound with a structure represented by the hydrophobic monomer formula (III-2) (where m=1):
[0129]
[0130] It is characterized in that the bacterial nitroreductase enzyme can reduce the terminal nitro group to hydroxylamine or primary amine, followed by 1,6-electron rearrangement to remove a molecule of carbon dioxide, and finally release a primary amine group.
[0131] Preparation method: Dissolve 4-nitrobenzyl alcohol (2.0g, 13.1mmol) and a catalytic amount of dibutyltin dilaurate (60μL) in 100mL of dry dichloromethane, and slowly add methanol dropwise at room temperature and stirring. Isocyanoethyl acrylate (3.1 g, 20.0 mmol). The reaction was finished after 4h, washed with saturated brine, dried with anhydrous magnesium sulfate, concentrated and purified by column chromatography to obtain a white solid, namely the compound of the structure represented by formula (III-2), the yield was 3.6g, and the yield was 90 %.
[0132] The compound of the str...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com