Preparation method for soluble amino phthalocyanine-titanium dioxide near-infrared photocatalyst
A near-infrared light, titanium dioxide technology, applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problem of inability to expand the near-infrared region, and achieve high catalytic Efficiency, combined with uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
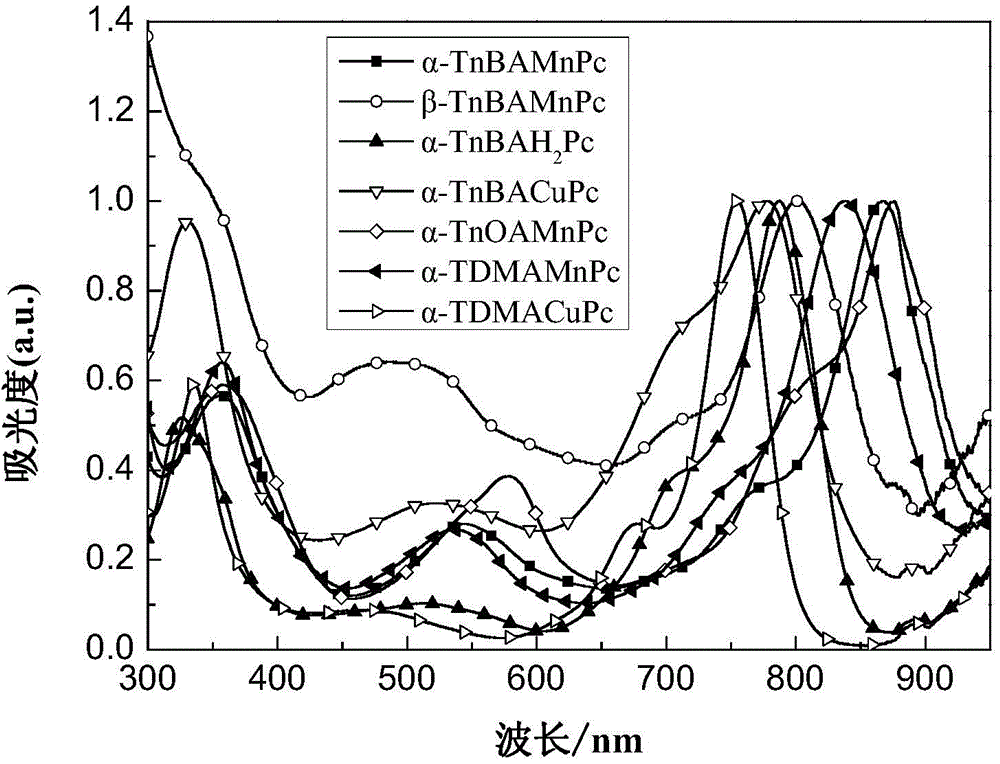
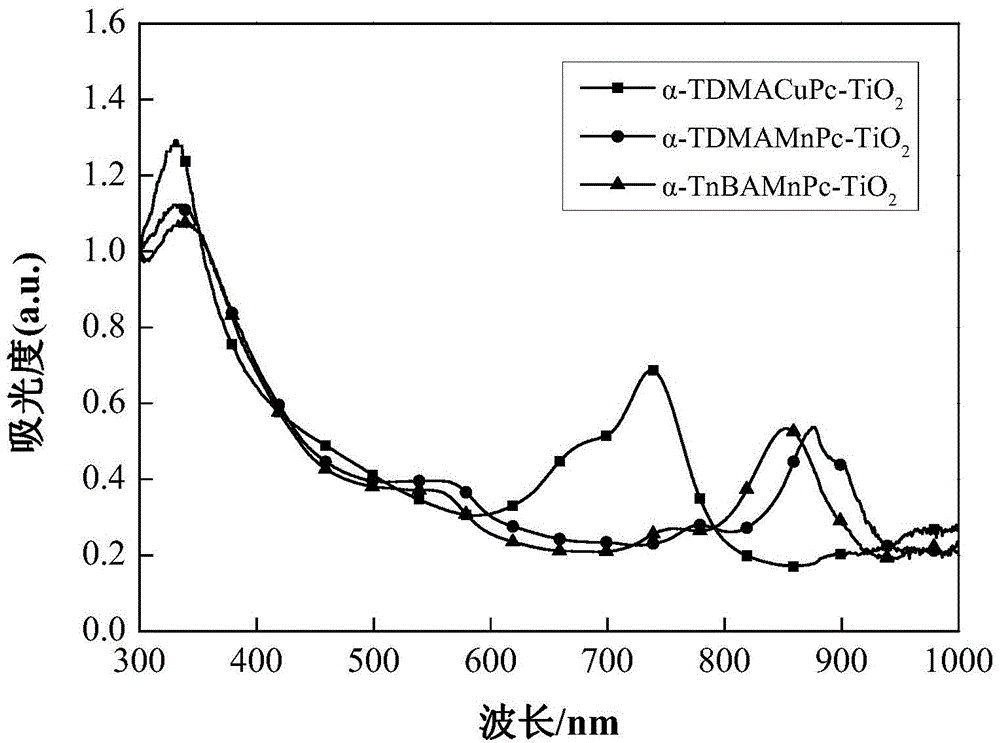
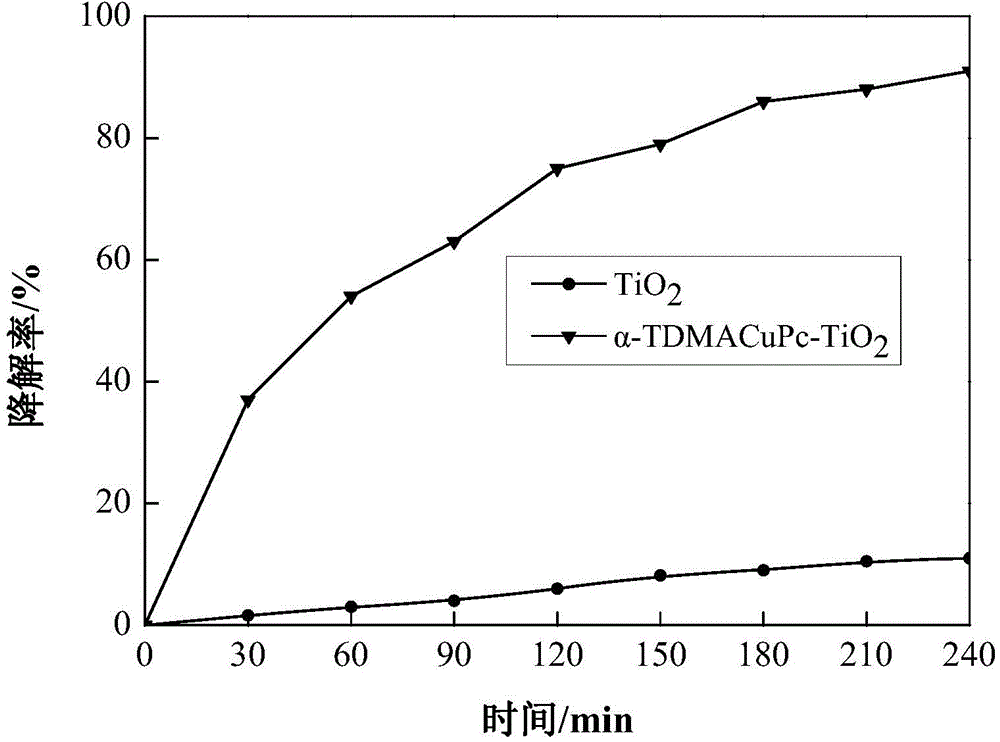
Image
Examples
Embodiment 1
[0029]The soluble amino phthalocyanine selected in this embodiment is 3,3', 3", 3"'-tetra-n-octylamine manganese phthalocyanine (α-TnOAMnPc), the specific structural formula is as follows, and the synthesis method is as follows:
[0030]
[0031] (1) Synthesis of 3-n-octylaminophthalonitrile (α-nOAPn):
[0032] 17.30g 3-nitrophthalonitrile, 14.19g n-octylamine, 20.24g triethylamine and 50mL DMAC were added successively to a 250mL three-necked flask equipped with a thermometer and a magnetic stirrer, and reacted at room temperature for 12h under stirring conditions. After the reaction is over, pour the reaction solution into a beaker filled with 500mL deionized water for precipitation, wash the precipitate with water until the washing solution is neutral, dry it, and recrystallize it with absolute ethanol to obtain an orange-yellow solid, which is dried in a vacuum oven at 50°C After 24 hours, 25.4 g of orange-yellow solid crystals were obtained, with a yield of 84%.
[003...
Embodiment 2
[0038] The soluble amino phthalocyanine selected in this embodiment is 4,4', 4 ", 4 "'-tetra-n-butylamino manganese phthalocyanine (β-TnBAMnPc), the specific structural formula is as follows, and the synthesis method is as follows:
[0039]
[0040] (1) Synthesis of 4-n-butylaminophthalonitrile (β-nBAPn):
[0041] In a 250mL three-necked flask equipped with a thermometer and a magnetic stirrer, 17.30g of 4-nitrophthalonitrile, 8.03g of n-butylamine, 20.24g of triethylamine and 50mL of DMAC were sequentially added, and reacted at room temperature for 12h under stirring conditions. After the reaction is over, pour the reaction solution into a beaker filled with 500mL deionized water for precipitation, wash the precipitate with water until the washing solution is neutral, dry it, and recrystallize it with absolute ethanol to obtain an orange-yellow solid, which is dried in a vacuum oven at 50°C After 24 hours, 22.9 g of orange-yellow solid crystals were obtained, with a yield ...
Embodiment 3
[0047] The soluble aminophthalocyanine selected in this embodiment is 3,3′,3″,3″′-tetradimethylaminocopper phthalocyanine (α-TDMACuPc), the specific structural formula is as follows, and the synthesis method is as follows:
[0048]
[0049] (1) Synthesis of 3-dimethylaminophthalonitrile (α-DMAPn):
[0050] In a 250mL three-necked flask equipped with a thermometer and a magnetic stirring bar, add 17.30g 3-nitrophthalonitrile, 20.45g dimethylamine aqueous solution (33wt%), 20.24g triethylamine and 50mL DMAC successively, under stirring condition Reaction at room temperature for 12h. After the reaction is over, pour the reaction solution into a beaker filled with 500mL deionized water to precipitate, wash the precipitate with water until the washing liquid is neutral, dry, and recrystallize with absolute ethanol to obtain an orange-yellow solid, which is put into a vacuum oven After drying at 50°C for 24 hours, 18.5 g of orange-yellow solid crystals were obtained, with a yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com