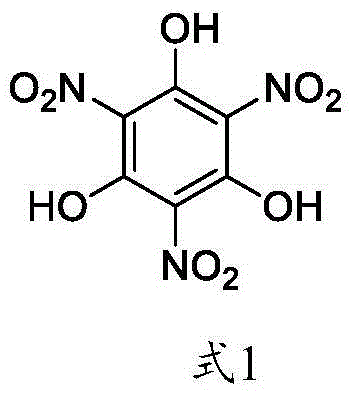
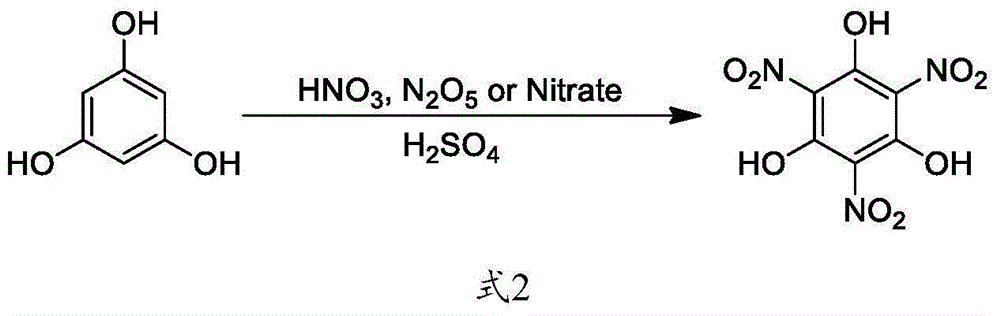
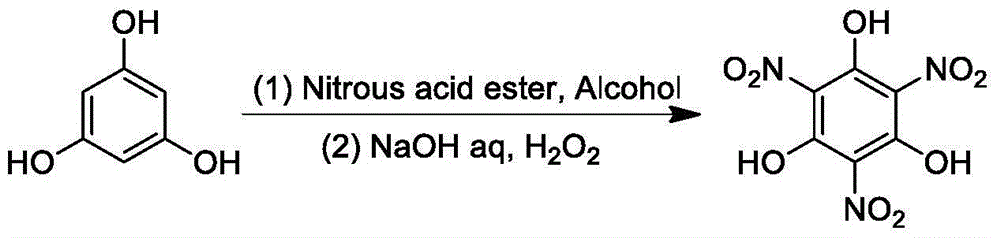Green synthetic method of trinitrophloroglucinol and application thereof
A technology of trinitrophloroglucinol and phloroglucinol, which is applied to the preparation of nitro compounds, nitrated explosive components, offensive equipment, etc., can solve the problems of strong corrosion, pollution, severe conditions, etc., and achieve low corrosion , Overcoming serious pollution and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]Weigh phloroglucinol (50mmoL), place it in a 250mL round bottom flask, add ethanol (80mL), and stir to dissolve. Under the condition of ice bath, measure n-propyl nitrite (167mmoL), slowly add it dropwise to the above reaction solution, stir in ice bath for 0.5h, rise to room temperature and stir for 0.5h. Ethanol was evaporated to dryness under reduced pressure, then 10% aqueous sodium hydroxide solution (120 mL) was added, stirred to dissolve, 30% hydrogen peroxide solution (300 mmoL) was measured, added dropwise to the above reaction solution, and stirred at room temperature for 8 h. Under ice-bath conditions, the pH of the reaction solution was adjusted to pH=1-2 with concentrated hydrochloric acid, extracted with ethyl acetate (50mL×3), the organic phases were combined, washed with water (50mL), and evaporated to dryness under reduced pressure to obtain yellow crystals Trinitrophloroglucinol, yield 77.5%.
[0044] The melting point of the obtained product is 165-16...
Embodiment 2
[0046] Weigh phloroglucinol (50mmoL), place it in a 250mL round bottom flask, add ethanol (80mL), and stir to dissolve. Under the condition of ice bath, measure n-propyl nitrite (150mmoL), slowly add dropwise to the above reaction solution, stir in ice bath for 0.5h, rise to room temperature and stir for 0.5h. Ethanol was evaporated to dryness under reduced pressure, then 10% aqueous sodium hydroxide solution (120 mL) was added, stirred to dissolve, 30% hydrogen peroxide solution (300 mmoL) was measured, added dropwise to the above reaction solution, and stirred at room temperature for 8 h. Under ice-bath conditions, the pH of the reaction solution was adjusted to pH=1-2 with concentrated hydrochloric acid, extracted with ethyl acetate (50mL×3), the organic phases were combined, washed with water (50mL), and evaporated to dryness under reduced pressure to obtain yellow crystals Trinitrophloroglucinol, yield 58.0%.
Embodiment 3
[0048] Weigh phloroglucinol (50mmoL), place it in a 250mL round bottom flask, add ethanol (80mL), and stir to dissolve. Under ice-bath conditions, measure n-propyl nitrite (200mmoL), slowly dropwise add to the above reaction solution, stir in ice-bath for 0.5h, rise to room temperature and stir for 0.5h. Ethanol was evaporated to dryness under reduced pressure, then 10% aqueous sodium hydroxide solution (120 mL) was added, stirred to dissolve, 30% hydrogen peroxide solution (300 mmoL) was measured, added dropwise to the above reaction solution, and stirred at room temperature for 8 h. Under ice-bath conditions, the pH of the reaction solution was adjusted to pH=1-2 with concentrated hydrochloric acid, extracted with ethyl acetate (50mL×3), the organic phases were combined, washed with water (50mL), and evaporated to dryness under reduced pressure to obtain yellow crystals Trinitrophloroglucinol, yield 78.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com