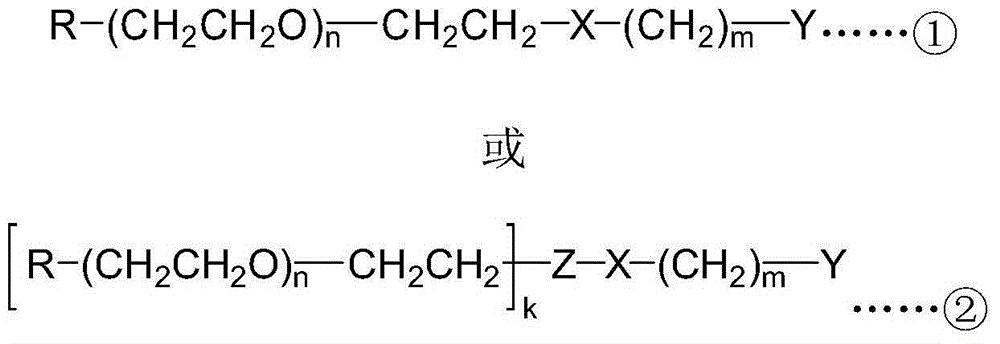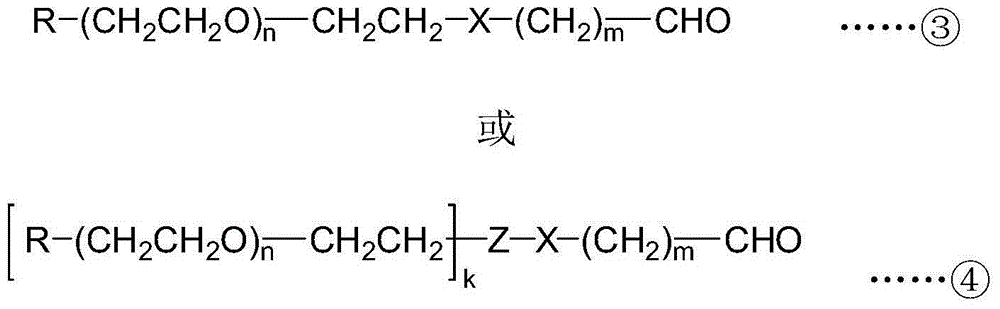Preparation method of high-purity polyethylene glycol aldehyde derivative
A technology of polyethylene glycol aldehyde and polyethylene glycol, applied in the field of preparation of high-purity polyethylene glycol aldehyde derivatives, can solve the problems of PEG chain decomposition, expensive raw materials, affecting the modification efficiency of protein and polypeptide drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
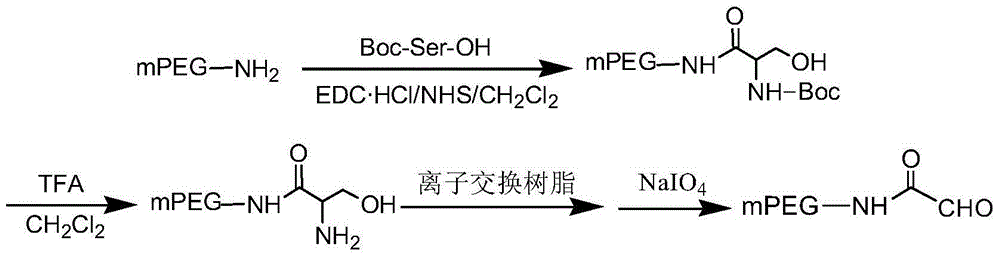
[0025] Embodiment 1: Preparation of monomethoxy polyethylene glycol 20000-aldehyde (mPEG20000-aldehyde)
[0026] The preparation method of high-purity polyethylene glycol aldehyde derivatives comprises the steps:
[0027] (1) Change the terminal hydroxyl group of PEG into a more active group to obtain a PEG active intermediate, and then use the PEG active intermediate to react with a small molecule compound having an α-hydroxy acid, β-amino alcohol or α-aminoketone structure A polyethylene glycol intermediate was obtained; specifically, the PEG active intermediate used in this example was prepared by converting the terminal hydroxyl group of a single-end blocked PEG into a group with higher activity. In this embodiment, monomethoxypolyethylene glycol (mPEG) is selected. The terminal hydroxyl group of monomethoxypolyethylene glycol 20000 (mPEG20000, with an average molecular mass of 20000) was converted into an amino group to obtain a PEG active intermediate, and then combined...
Embodiment 2
[0038] Embodiment 2: the preparation of monomethoxy polyethylene glycol 5000-propionaldehyde (mPEG5000-propionaldehyde)
[0039] The preparation method of high-purity polyethylene glycol aldehyde derivatives comprises the steps:
[0040] (1) The terminal hydroxyl group of monomethoxypolyethylene glycol 5000 (mPEG5000, the average relative molecular mass is 5000) is converted into p-toluenesulfonate to obtain monomethoxypolyethylene glycol 5000-p-toluenesulfonate ( mPEG5000-OTs), then react with α-hydroxyl-γ-aminobutyric acid to obtain polyethylene glycol intermediate monomethoxy polyethylene glycol 5000-hydroxy acid (mPEG5000-hydroxy acid), its structural formula is Wherein n is 113 on average, m is 2, R is a methoxyl group, X is an amino bond, and Y is an α-hydroxy acid structure.
[0041] (2) use anion exchange resin to separate and purify the polyethylene glycol intermediate;
[0042] (3) Polyethylene glycol intermediate is used sodium periodate (NaIO 4 ) to oxidize it ...
Embodiment 3
[0053] Embodiment 3: Preparation of double-chain monomethoxy polyethylene glycol 5000-lysine-acetaldehyde (2mPEG5000-Lys-acetaldehyde)
[0054] The preparation method of high-purity polyethylene glycol aldehyde derivatives comprises the steps:
[0055] (1) The PEG active intermediate selected in this embodiment is double-chain monomethoxypolyethylene glycol 5000-lysine-succinimide ester (2mPEG5000-Lys-NHS, the average value of each polyethylene glycol chain The relative molecular mass is 5000), and it is reacted with the small molecule compound 3-amino-2-hydroxypropionic acid to obtain the polyethylene glycol intermediate 2mPEG5000-Lys-hydroxy acid, and its structural formula is Wherein n is 113 on average, m is 1, k is 2, R is a methoxy group, X is an amide bond, Y is an α-hydroxy acid structure, and Z is lysine (Lys).
[0056] (2) use anion exchange resin to separate and purify the polyethylene glycol intermediate;
[0057] (3) Polyethylene glycol intermediate is used sod...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap