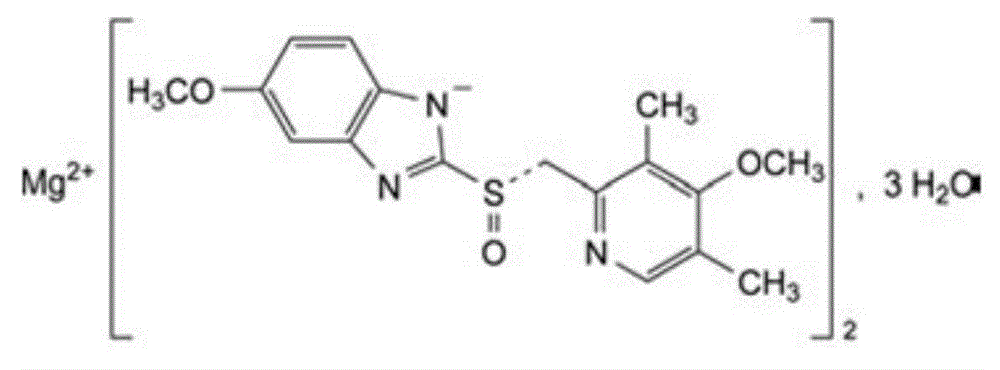
Esomeprazole magnesium enteric capsule and preparation method thereof
A technology for enteric coating of esomeprazole magnesium and esomeprazole, which is applied in the field of pharmaceutical preparations, can solve the problems of poor solubility of esomeprazole magnesium, influence on stability, influence on illumination, etc. Ensure safety and stable blood concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] An enteric-coated capsule of esomeprazole magnesium, which is made by mixing and filling immediate-release enteric-coated pellets and sustained-release enteric-coated pellets, and the composition of each component is as follows by weight percentage: immediate-release enteric-coated pellets 13.0% of pellets, 87.0% of slow-release enteric-coated pellets; both the quick-release enteric-coated pellets and the slow-release enteric-coated pellets include a core, a drug-coated layer, an isolation layer, and an enteric-coated layer.
[0023] The drug layer formula of the immediate-release enteric-coated pellets: microcrystalline cellulose seed core: 50g, esomeprazole magnesium: 400g, hypromellose: 133g, purified water: 1771g. Immediate-release enteric-coated pellets isolation layer formula: upper drug layer pellets: 250g, hydroxypropyl cellulose: 21g, talcum powder: 38g, magnesium stearate: 3g, purified water: 522g. Enteric-coated layer formula of immediate-release enteric-coat...
Embodiment 2
[0028] An enteric-coated capsule of esomeprazole magnesium, which is made by mixing and filling immediate-release enteric-coated pellets and sustained-release enteric-coated pellets, and the composition of each component is as follows by weight percentage: immediate-release enteric-coated pellets 32.0% of pellets, 68.0% of sustained-release enteric-coated pellets; both the rapid-release enteric-coated pellets and the sustained-release enteric-coated pellets include a pellet core, a drug-coated layer, an isolation layer, and an enteric-coated layer.
[0029] Drug layer formula of immediate-release enteric-coated pellets: microcrystalline cellulose seed core: 250g, esomeprazole magnesium: 500g, hypromellose: 167g, purified water: 2214g. The formulation of the isolation layer of the immediate-release enteric-coated pellets: upper drug layer pellets: 250g, hydroxypropyl cellulose: 19g, talcum powder: 34g, magnesium stearate: 3g, purified water: 466g. Enteric-coated layer formula o...
Embodiment 3
[0034] An enteric-coated capsule of esomeprazole magnesium, which is made by mixing and filling immediate-release enteric-coated pellets and sustained-release enteric-coated pellets, and the composition of each component is as follows by weight percentage: immediate-release enteric-coated pellets 51.0% of pellets, 49.0% of sustained-release enteric-coated pellets; both the rapid-release enteric-coated pellets and the sustained-release enteric-coated pellets include a core, a drug-coated layer, an isolation layer, and an enteric-coated layer.
[0035] Drug layer formula of immediate-release enteric-coated pellets: microcrystalline cellulose seed core: 250g, esomeprazole magnesium: 600g, hypromellose: 200g, purified water: 2657g. Immediate-release enteric-coated pellets isolation layer formula: upper drug layer pellets: 250g, hydroxypropyl cellulose: 16g, talcum powder: 29g, magnesium stearate: 2g, purified water: 395g. Enteric-coated layer formula of immediate-release enteric-coa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com