Trifluoromethylthio reagent as well as synthetic method and application thereof
A technology of trifluoromethylthio group and reagent, applied in the field of trifluoromethylthio group reagent, can solve the problems of harsh reaction conditions, low conversion rate, unsuitable for industrial production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
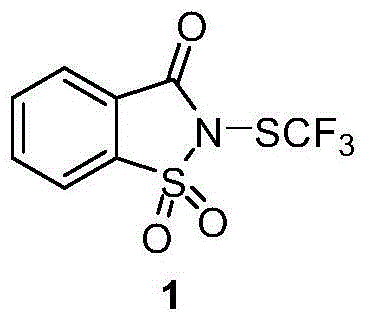
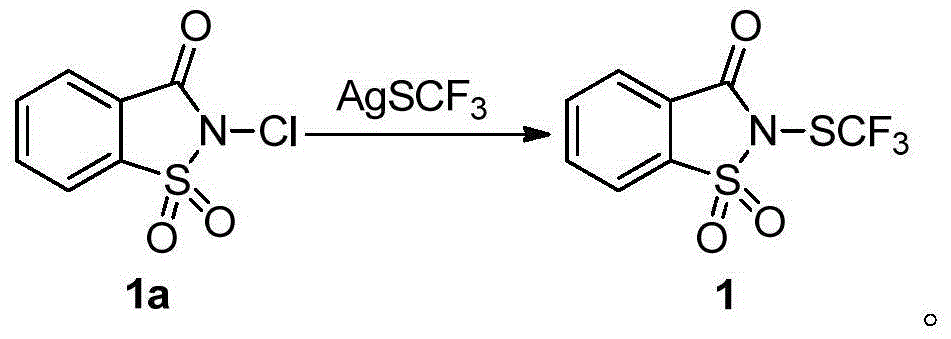
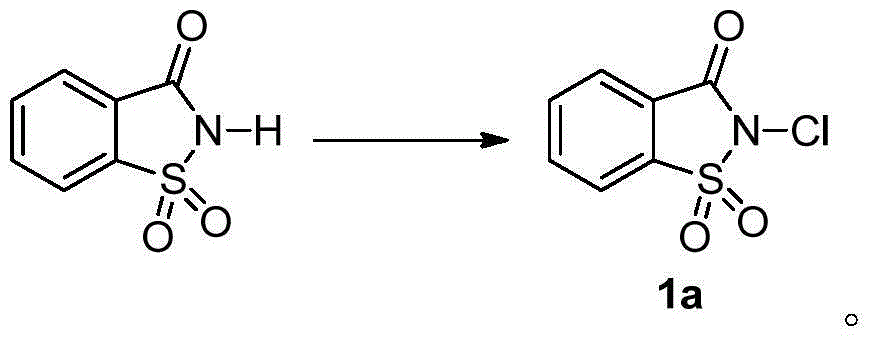
[0085] Example 1 Synthesis of trifluoromethylthiolation reagent 1.
[0086] Saccharin (6.0g) was reacted with tert-butyl hypochlorite (5ml) in methanol (120ml) at room temperature for 5 minutes to obtain compound chlorosaccharin 1a (6.0g, 84%); Silver fluoromethylthio (3.6g) was reacted in acetonitrile (40ml) at room temperature for 10 minutes to obtain compound 1 (3.3g, 86%). Reagent 1 is a white solid at room temperature, soluble in dichloromethane, chloroform, acetone, acetonitrile and other organic solvents.
[0087]
[0088] Nitrogen-trifluoromethylthiosaccharin (2-((Trifluoromethyl)thio)benzo[d]isothiazol-3(2H)-one1,1-dioxide): 1 H NMR (400MHz, cdcl 3 )δ8.20(d,J=7.6Hz,1H),8.06-7.97(m,2H),7.96-7.90(m,1H); 19 F NMR (376MHz, CDCl 3 )δ-47.34(s,3F); 13 C (126MHz, CDCl 3 )δ158.50,138.08,136.50,135.12,127.42(q,J=31.6Hz),126.67,126.30,122.12ppm.MS(DART POS):283.97(M+H);HRMS(DART POS):C 8 h 5 o 3 NF 3 S 2 (M+H) calculated value: 283.9657, experimental value: 283.965...
Embodiment 2
[0091]
[0092] Dissolve 1-methylbenzylamine (37mg, 0.3mmol) and trifluoromethylthiolation reagent (100mg, 0.36mmol) in dichloromethane (6.0mL) under air condition, stir at room temperature for 1 hour, then add 100μl triethyl The amine was evaporated under reduced pressure to remove the solvent, and the residue was purified by a flash silica gel column to obtain the corresponding nitrogen trifluoromethylthioated product a1 (60 mg, 90%). The purity is greater than 95% identified by hydrogen spectrum.
[0093] Nitrogen-trifluoromethylthio-1-phenyl-ethylamine ((S)-N-(1-Phenylethyl)-S-(trifluoromethyl)thiohydroxylamine): 1 H NMR (400MHz, CDCl 3 )δ7.39–7.36(m,2H),7.33–7.30(m,3H),4.24(q,J=6.6Hz,1H),3.28(s,1H),1.50(d,J=6.6Hz,3H )ppm; 19 F NMR (376MHz, CDC 3 )δ-51.86(s,3F)ppm; 13 C NMR (126MHz, CDCl 3 )δ143.41,130.38(q,J=317.4Hz),128.67,127.85,126.70,60.61,22.54ppm.MS(EI):221.1;HRMS(EI):C 9 h 10 f 3 NS calculated value: 221.0486, experimental value: 221.0487. IR: υ3354, 30...
Embodiment 3
[0095]
[0096] Dissolve phenethylamine (37mg, 0.3mmol) and trifluoromethylthiolation reagent (100mg, 0.36mmol) in dichloromethane (6.0mL) under air conditions, stir at room temperature for 1 hour, add 100μl triethylamine, reduce The solvent was removed by rotary evaporation under high pressure, and the residue was purified by a flash silica gel column to obtain the corresponding nitrogen trifluoromethylthioated product a2 (63 mg, 94%). The purity is greater than 95% identified by hydrogen spectrum.
[0097] Nitrogen-trifluoromethylthio-2-phenyl-ethylamine (N-Phenethyl-S-(trifluoromethyl)thiohydroxylamine): 1 H NMR (400MHz, CDCl 3 )δ7.36-7.30(m,2H),7.28-7.23(m,1H),7.22-7.17(m,2H),3.36(td,J=6.8,6.0Hz,2H),2.89(br,NH, 1H),2.86(t,J=7.0Hz,2H)ppm; 19 F NMR (376MHz, CDCl 3 )δ-52.47(s,3F)ppm; 13 C NMR (126MHz, CDCl 3 )δ138.42,130.40(q,J=317.5Hz),128.76,128.65,126.54,54.62,36.60ppm.MS(EI):221.0;HRMS(EI):C 9 h 10 NF 3 S calculated value: 221.0486, experimental value: 221.048...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com