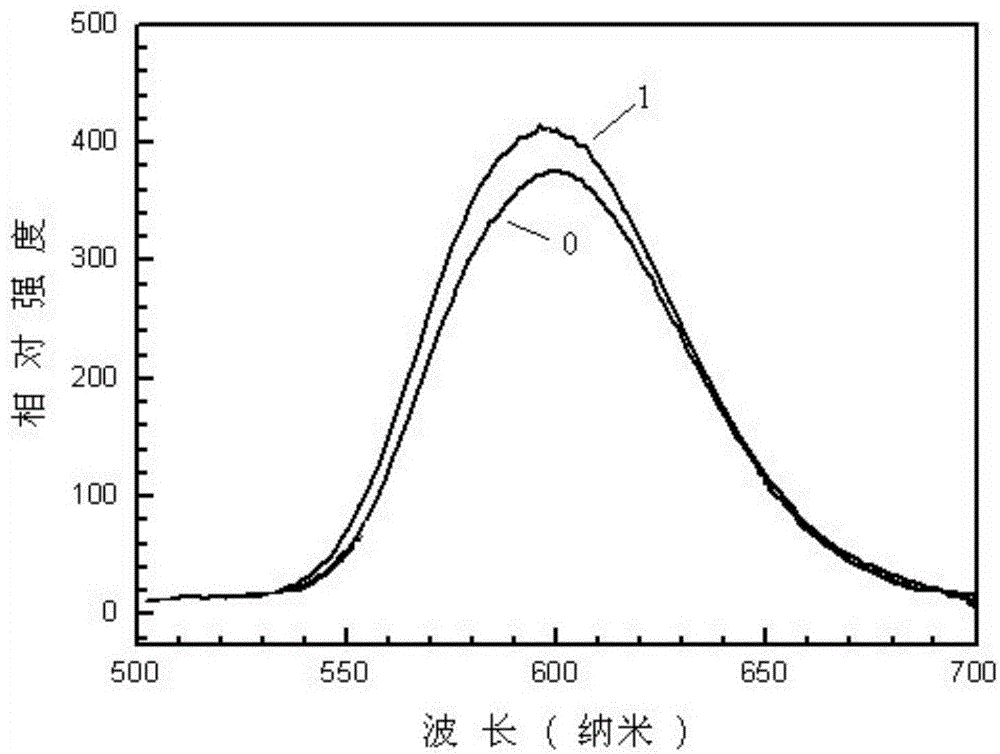
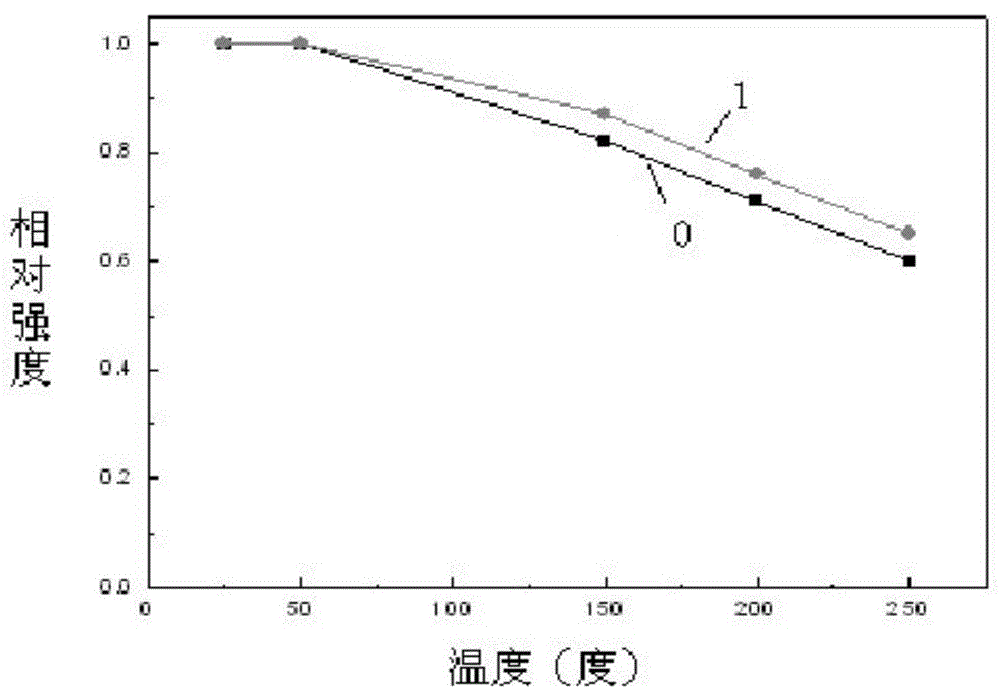
High-luminous decay resistance nitride and nitrogen oxide fluorescent materials and preparation method thereof
A nitrogen oxide and fluorescent material technology, applied in the field of fluorescent materials, can solve the problems of large uncertainty of metering ratio, large fluctuation of material luminous brightness and chromaticity coordinates, and narrow half-peak width of luminous efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] raw material
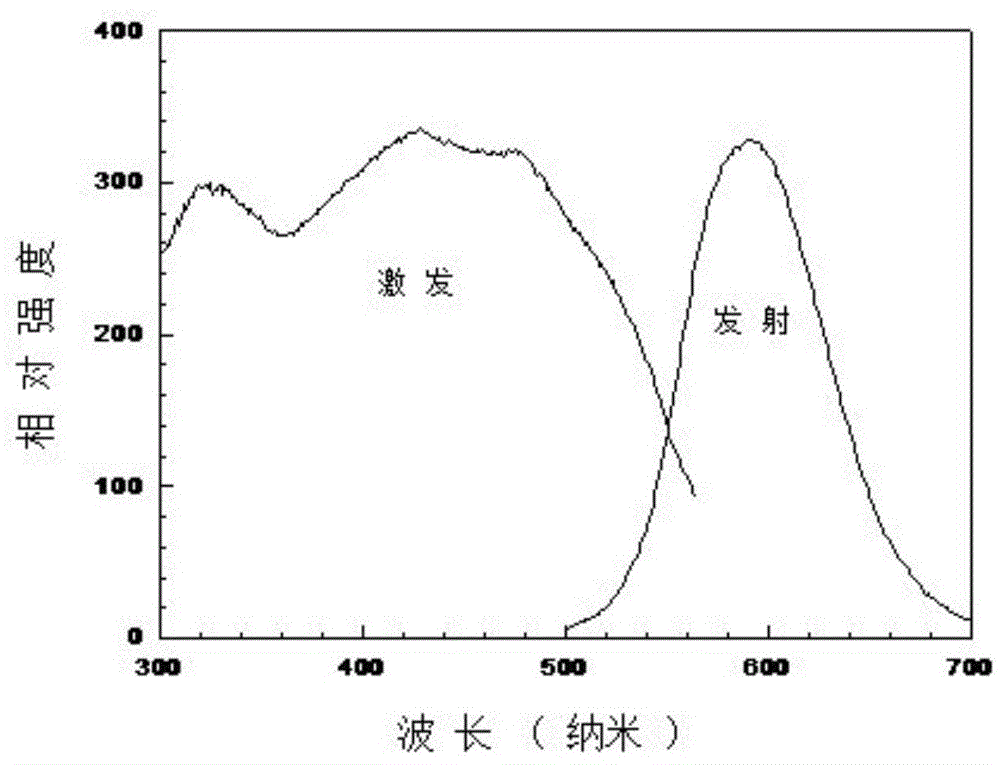
[0059] First, under the protection of inert gas, the metal elemental Sr and Eu are treated in flowing nitrogen at a temperature of 500 ° C to 1000 ° C to obtain Sr 3 N 2 and EuN precursor nitride, and then the above raw materials were weighed in a molar ratio in an inert gas-protected glove box, and then thoroughly ground and mixed uniformly. The mixture is then loaded into a boron nitride or molybdenum crucible, placed in a gas pressure sintering furnace, under N 2 Calcination in atmosphere at 0-5 atmospheric pressure, 1200-1600°C for 6 hours. After the sintered body is cooled, it is pulverized and ground to obtain the silicon carbide-enhanced composite fluorescent material Sr with red luminescence in the present invention 1.95 Si 5 N 7.6 C 0.4 : 0.05Eu 2+ , 0.03F - 0.3Ag. figure 1 It is the excitation and emission spectrum diagram of the fluorescent material of Example 1, and its emission wavelength is at 600nm.
Embodiment 2
[0064] raw material
[0065] First, under the protection of an inert gas, the metal elemental Ca and Eu are treated in flowing nitrogen at a temperature of 500 ° C to 1000 ° C to obtain Ca 3 N 2 and EuN precursor nitride, and then the above raw materials were weighed in a molar ratio in an inert gas-protected glove box, and then thoroughly ground and mixed uniformly. The mixture is then loaded into a boron nitride or molybdenum crucible, placed in a gas pressure sintering furnace, under N 2 Calcination in atmosphere at 0-10 atmospheric pressure, 1500-1800°C for 6 hours. After the sintered body is cooled, it is pulverized and ground to obtain the multi-phase fluorescent material Ca enhanced by silicon carbide with red light emission in the present invention. 0.99 AlSiN 2.85 C 0.15 : 0.01Eu 2+ , 0.03F - · 0.15Ag. Figure 4 It is the excitation and emission spectrum diagram of the fluorescent material of embodiment 2, and its emission wavelength is at 650nm.
Embodiment 3
[0070] raw material
[0071] Ag
[0072] Firstly, after weighing the above raw materials according to the molar ratio in an inert gas-protected glove box, they are fully ground and mixed evenly. The mixture is then loaded into a boron nitride or molybdenum crucible, placed in a gas pressure sintering furnace, under N 2 Calcination in the atmosphere at 5-10 atmospheric pressure, 1800-2200°C for 3 hours. After the sintered body is cooled, it is pulverized and ground to obtain the SiC-enhanced composite fluorescent material Si with green luminescence in the present invention. 5.75 Al 0.25 o 0.25 N 7.65 C 0.1 : 0.001Eu 2+ · 0.15Ag. Figure 7 It is the excitation and emission spectrum diagram of the fluorescent material of embodiment 3, and its emission wavelength is at 538nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com