Beta-amyloid 1-42 oligomer as well as preparation method and identification method therefor
An oligomer and tetramer technology, applied in the field of identification, Aβ1-42 oligomer and its preparation, can solve the problems of complex composition, unstable oligomer, low yield and the like, and achieve single composition and preparation method. Simple, high-yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] A method for preparing Aβ1-42 oligomers, comprising the following steps:
[0064] 1. Add 1 mg of Aβ1-42 synthetic peptide to 220 μL HFIP, and let it stand at room temperature for 30 minutes to obtain a completely dissolved solution;
[0065] 2. Transfer the solution obtained in step 1 to a siliconized centrifuge tube, and blow it on a dry bath nitrogen blower for 8 minutes to completely dry the HFIP, and obtain a transparent sheet-like solid that sinks to the bottom of the tube;
[0066] 3. Add 40 μl DMSO to the centrifuge tube to dissolve the solid at the bottom of the centrifuge tube completely, then add a 10 mM HEPES buffer solution with a pH value of 7.4 to make the final final volume of the solution 1 ml, mix well, and get final product.
Embodiment 2
[0068] A method for preparing Aβ1-42 oligomers, comprising the following steps:
[0069] 1. Add 1 mg of Aβ1-42 synthetic peptide to 225 μL HFIP, and let stand at room temperature for 60 minutes to obtain a completely dissolved solution;
[0070] 2. Transfer the solution obtained in step 1 to a siliconized centrifuge tube, and blow it on a dry bath nitrogen blower for 15 minutes to completely dry the HFIP, and obtain a transparent sheet-like solid that sinks to the bottom of the tube;
[0071] 3. Add 40 μl DMSO to the centrifuge tube to dissolve the solid at the bottom of the centrifuge tube completely, then add a 10 mM HEPES buffer solution with a pH value of 7.4 to make the final final volume of the solution 1 ml, mix well, and get final product.
[0072] 3. Identification method of Aβ1-42 oligomer
Embodiment 3
[0074] A method for identifying Aβ1-42 oligomers, comprising the following steps:
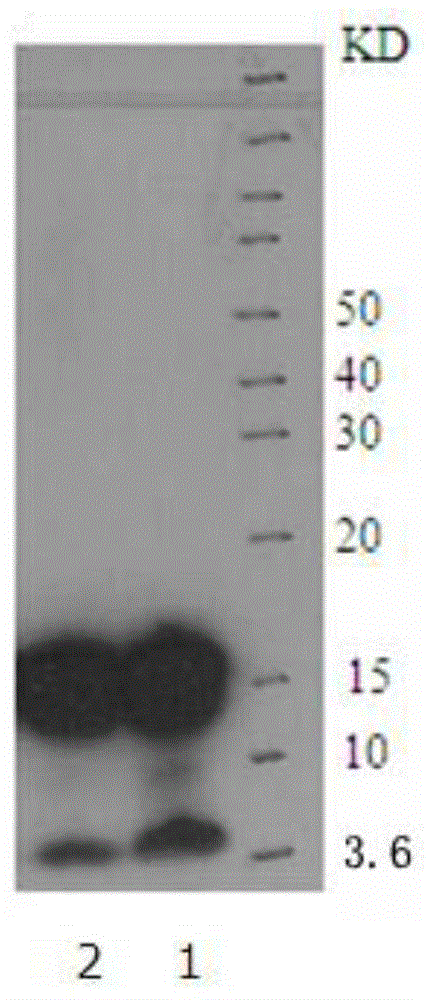
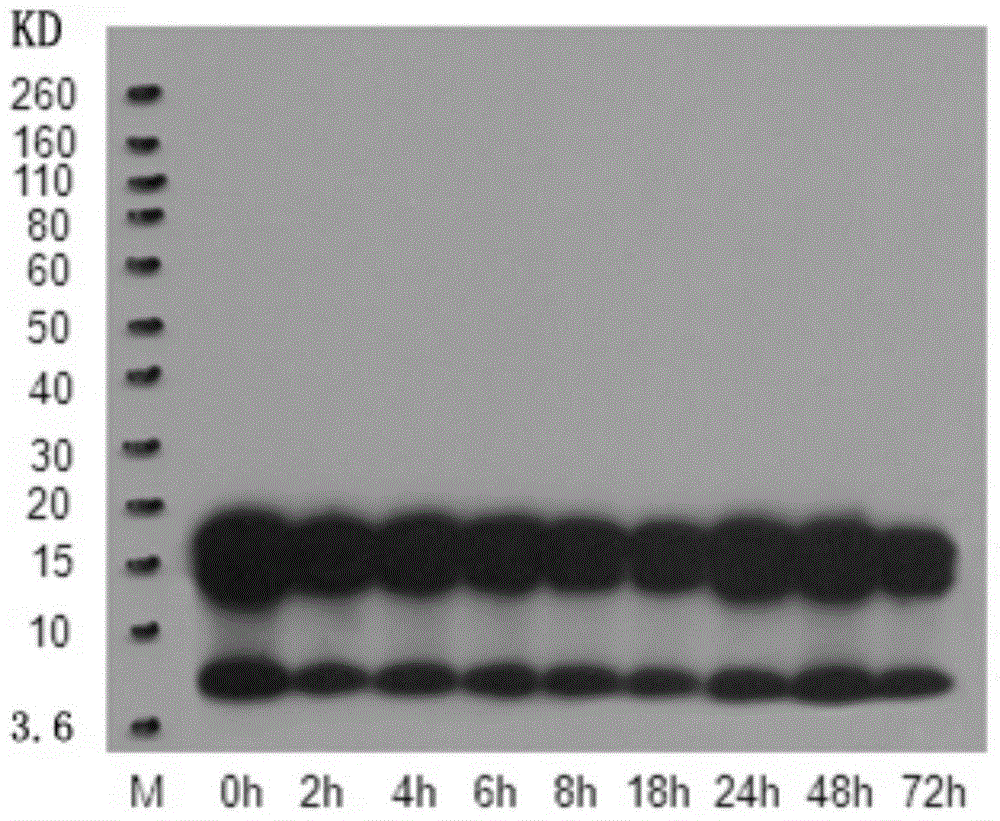
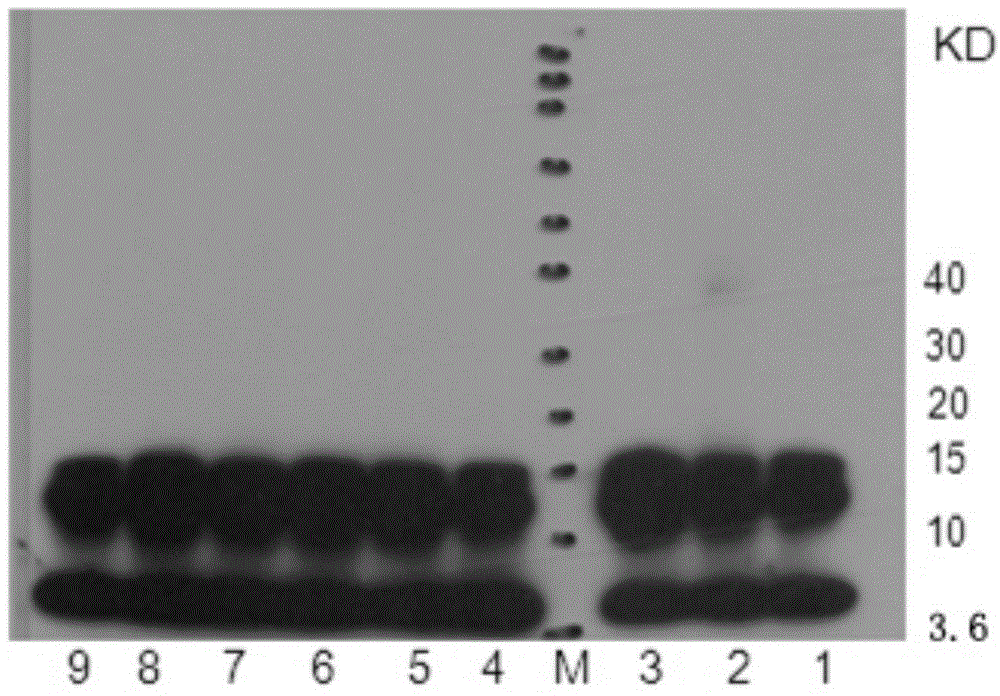
[0075] 1. SDS-polyacrylamide gel electrophoresis: Take 2 μL of the Aβ1-42 oligomer solution obtained in Example 1, mix it with 18 μL 4X electrophoresis sample buffer, and then load the sample. The electrophoresis sample volume is 5ul / swimming lane. The gel used for electrophoresis is 4–12% gradient gel, and 10 μL of pre-stained molecular weight standard with a molecular weight of 3.6-260KD is added to one lane, and the voltage of 80V is selected for electrophoresis for 30 minutes, and then the voltage of 100v is used to continue electrophoresis for 2 hours;
[0076] 2. Membrane transfer: transfer the electrophoresis protein band to the PVDF membrane, transfer the membrane by semi-dry method, the time is 6 minutes, cut the transferred PVDF membrane according to the size of the sample lane, and then soak the cut PVDF membrane into the TBST solution for 5 minutes;
[0077] 3. Sealing: Soak the PV...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com