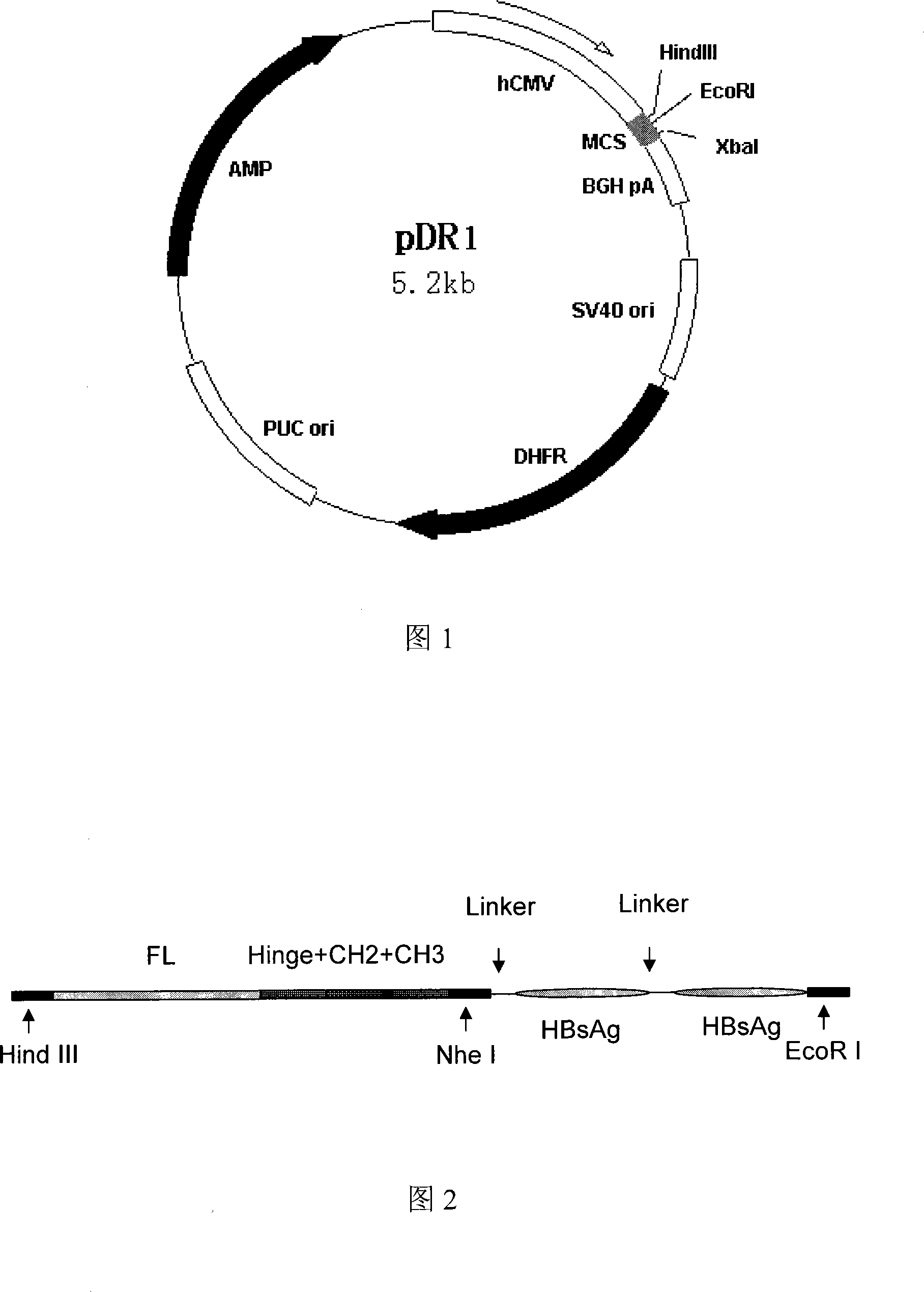
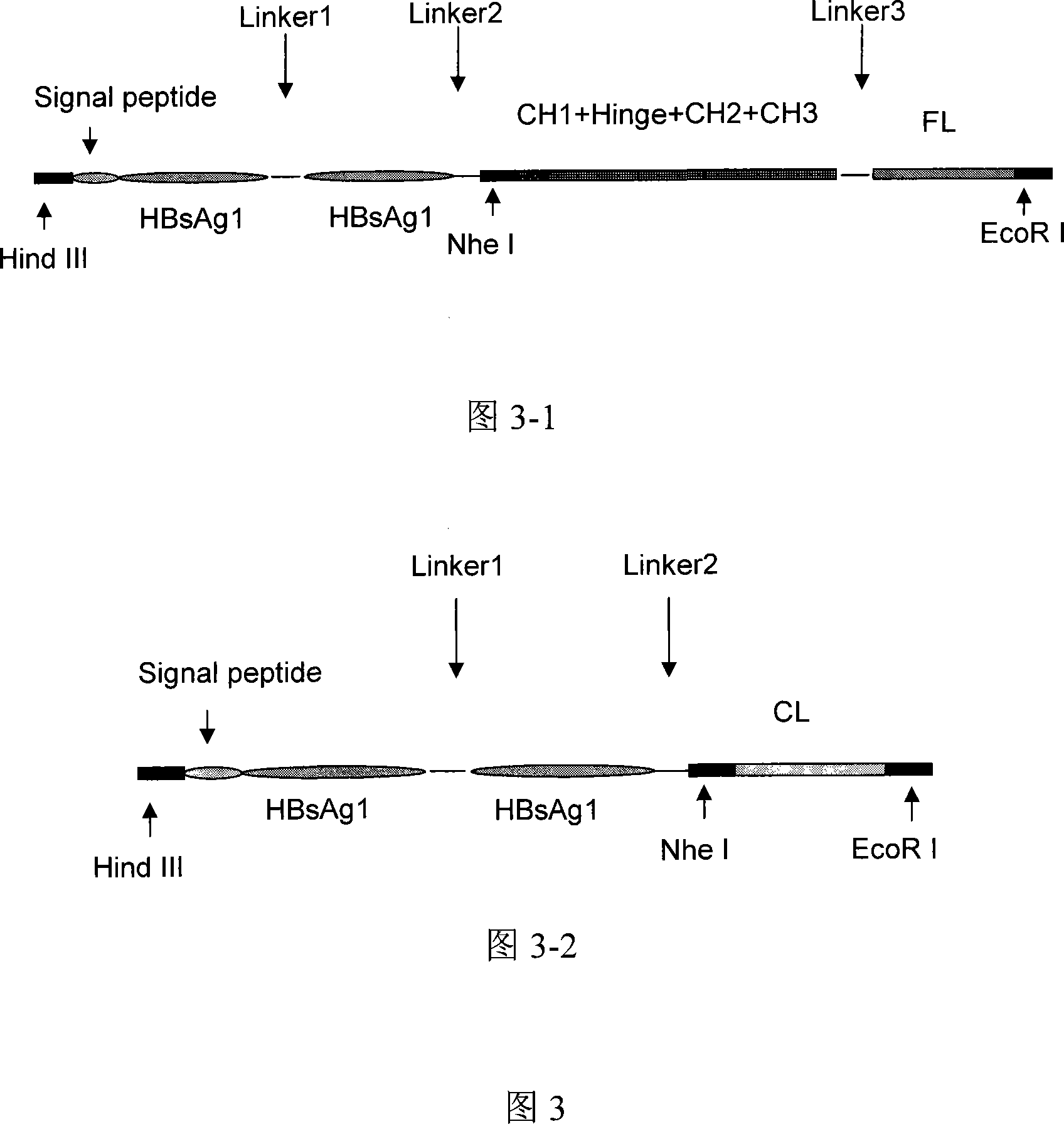
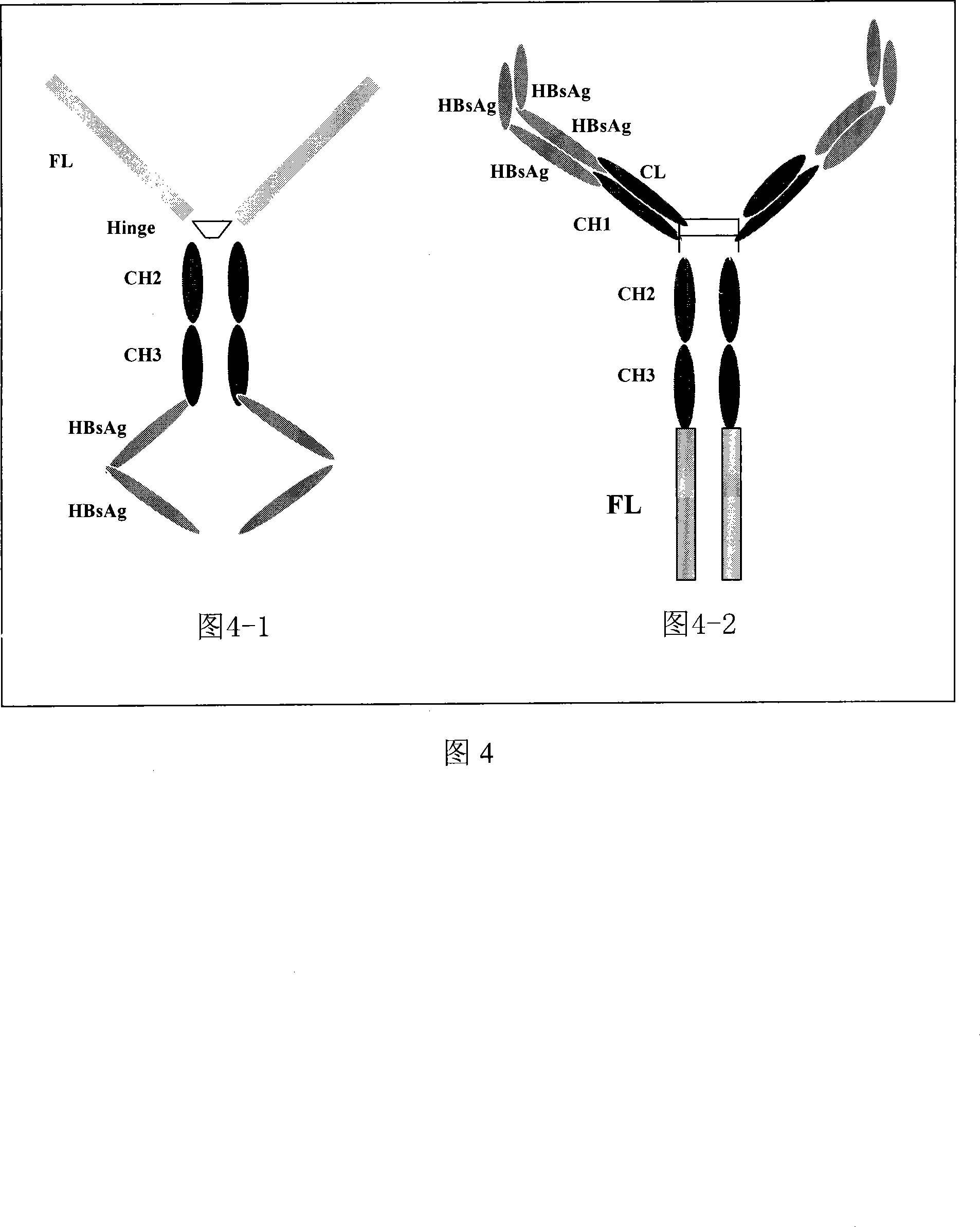
Hepatitis B fusion protein multiva lentvaccine, preparation method and uses thereof
A fusion protein, hepatitis B technology, applied in the field of multivalent vaccine preparation, can solve the problems of pollution, difficult quality inspection standards, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1. PCR of N-terminal fusion Flt3 ligand extracellular segment gene
[0046] Referring to the information and sequence of human Flt3 ligand provided by GENEBANK, synthetic primers: FL primer sense (primer 1): GCG ATGACAGTGCTGGCGCCA (Hind III) and FL antisense (primer 2): CGGGGCTGTCGGGGCTGT (Shaded part is complementary to Hinge 5'; underlined part is complementary to FL 3'). Human T lymphocytes were isolated, RNA was extracted with TRISOL kit (INVITROGEN), and a 546bp DNA fragment was obtained by RT-PCR (PROMEGA). After the fragments were recovered by a gel recovery kit (Shanghai Sangong), they were used for later use. The nucleotide sequence of the Flt3 ligand extracellular segment gene is shown in SEQ ID NO.1.
Embodiment 2
[0047] Example 2. PCR of human antibody heavy chain constant region Hinge+CH2+CH3 gene
[0048] Use lymphocyte separation medium to separate healthy human lymphocytes, use Trizol reagent (Invitrogen company product) to extract total RNA, and design primers for the Hinge+CH2+CH3 gene of the constant region of the heavy chain of the human antibody: Hinge+CH2+CH3 sense (primer 3) : GAG CCC AAA TCT TGT GAC (this sequence is consistent with Hinge5') and Hinge+CH2+CH3 antisense (primer 4): GCG TTT ACC CGG AGA CAGGGA (Nhe I, this sequence is complementary to CH3 3'), amplifies the antibody heavy chain constant region gene 696bp. The PCR reaction adopts hot start, and the reaction conditions are: 94°C for minutes; 94°C for 45 seconds, 60°C for 45 seconds, 72°C for 1 minute and 10 seconds, 30 cycles; 72°C for 10 minutes. The PCR products were purified and recovered by agarose gel electrophoresis. The heavy chain CH2-CH3 gene sequence of human IgG1 with hinge region is shown in SEQ I...
Embodiment 3
[0049] Example 3. Cloning of FL-Hinge-CH2-CH3
[0050] FL and Hinge+CH2+CH3 were used as templates, FL sense (primer 1) and Hinge+CH2+CH3 antisense (primer 4), amplified by overlap PCR to obtain FL+Hinge+CH2+CH3 (1242bp), and cloned into In the pGEM-T vector, it was confirmed that the correct clone was obtained after sequencing verification. The correct clone in this example was designated pGEM-T / FL-Hinge-CH2-CH3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com