Method for separating and purifying m-trihydroxybenzene compounds in Agrimonia polosa Ledeb
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
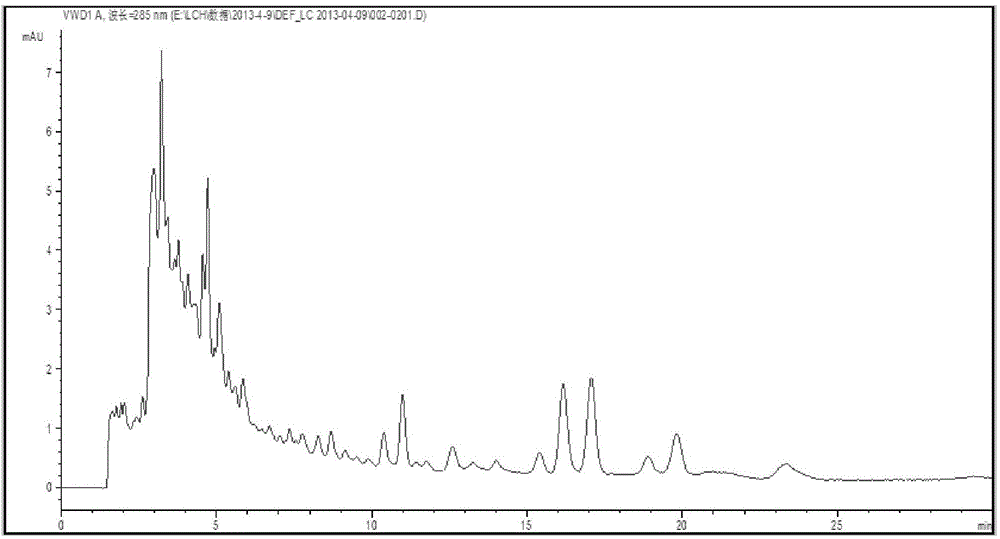
[0040] 1. Preparation of the crude extract of Agrimony: take 150g of Agrimony and crush it into a coarse powder, add 1500ml of petroleum ether (60-90°C), heat and reflux for extraction twice, each time for 1h; filter, combine the filtrates, recover the solvent and residue Add 50ml of chloroform to dissolve, shake and extract with 50ml of 5% sodium hydroxide, discard the chloroform solution, adjust the pH value of the sodium hydroxide solution to 1-2 with dilute hydrochloric acid, shake and extract twice with chloroform, combine the chloroform solution, and wash with water , discarded the aqueous solution, and concentrated the chloroform solution to obtain 310 mg of Agrimony crude extract. High performance liquid chromatography as figure 1 shown.
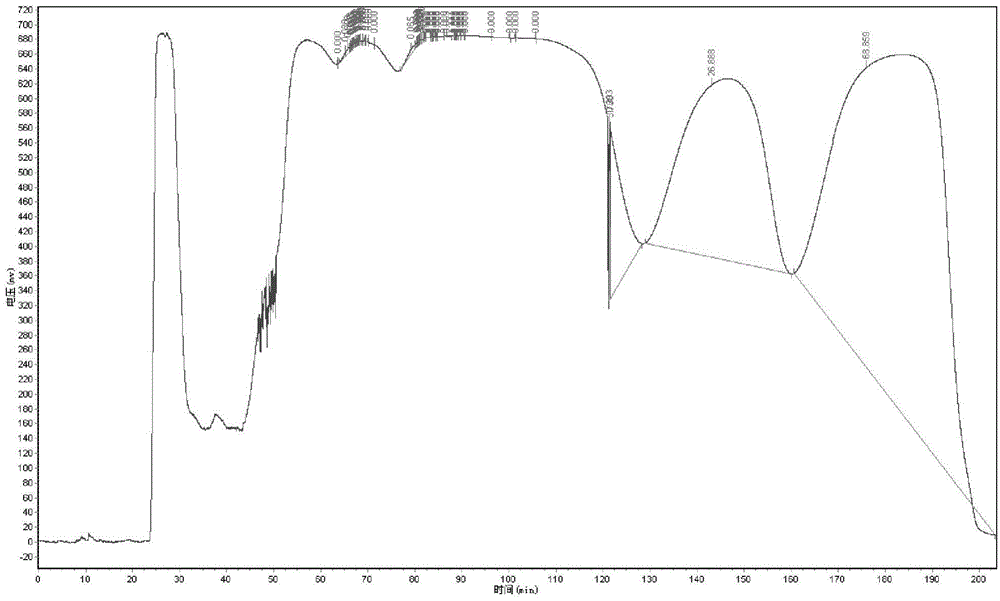
[0041] 2. The first high-speed countercurrent chromatographic separation: make n-hexane, acetonitrile, dichloromethane, and methanol into a solvent system in a volume ratio of 6:6:0.5:0.5, mix evenly, let stand to separate layers, a...
Embodiment 2
[0053] 1. Preparation of Agrimony Crude Extract: Steps are the same as in Example 1.
[0054] 2. The first high-speed countercurrent chromatographic separation: the solvent system is composed of n-hexane, acetonitrile and dichloromethane with a volume ratio of 5:5:0.5, the column temperature is set at 10°C, the rotation speed is 700rpm, and the flow rate of the mobile phase is 2mL / min, the 140-170 min eluate was collected in separate tubes at 8 min gradient time intervals, and the remaining steps of high-speed countercurrent chromatographic separation were the same as in Example 1.
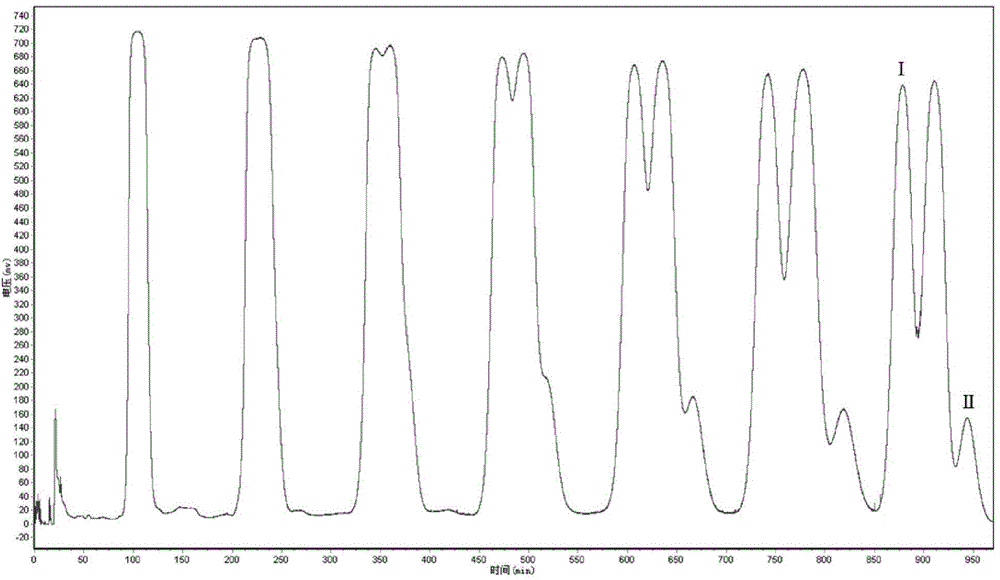
[0055] 3. The second high-speed countercurrent chromatographic separation: the solvent system is composed of n-hexane, dichloromethane and acetonitrile with a volume ratio of 10:7:3. min, the remaining steps of high-speed countercurrent chromatographic separation are the same as in Example 1.
Embodiment 3
[0057] 1. Preparation of Agrimony Crude Extract: Steps are the same as in Example 1.
[0058] 2. The first high-speed countercurrent chromatographic separation: The solvent system is composed of petroleum ether, acetonitrile, dichloromethane and methanol with a volume ratio of 6:6:0.5:0.5. The column temperature is set at 20°C and the speed is 1000rpm. The flow rate was 5mL / min, and the eluate was collected in separate tubes at 3min gradient time intervals for 65-90min. The remaining steps of high-speed countercurrent chromatographic separation were the same as in Example 1.
[0059] 3. The second high-speed countercurrent chromatography separation: the solvent system is composed of n-hexane, dichloromethane and acetonitrile with a volume ratio of 10:7:3, the column temperature is set at 20°C, the rotation speed is 700rpm, and the flow rate of the mobile phase is 5mL / min, the remaining steps of high-speed countercurrent chromatographic separation are the same as in Example 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com