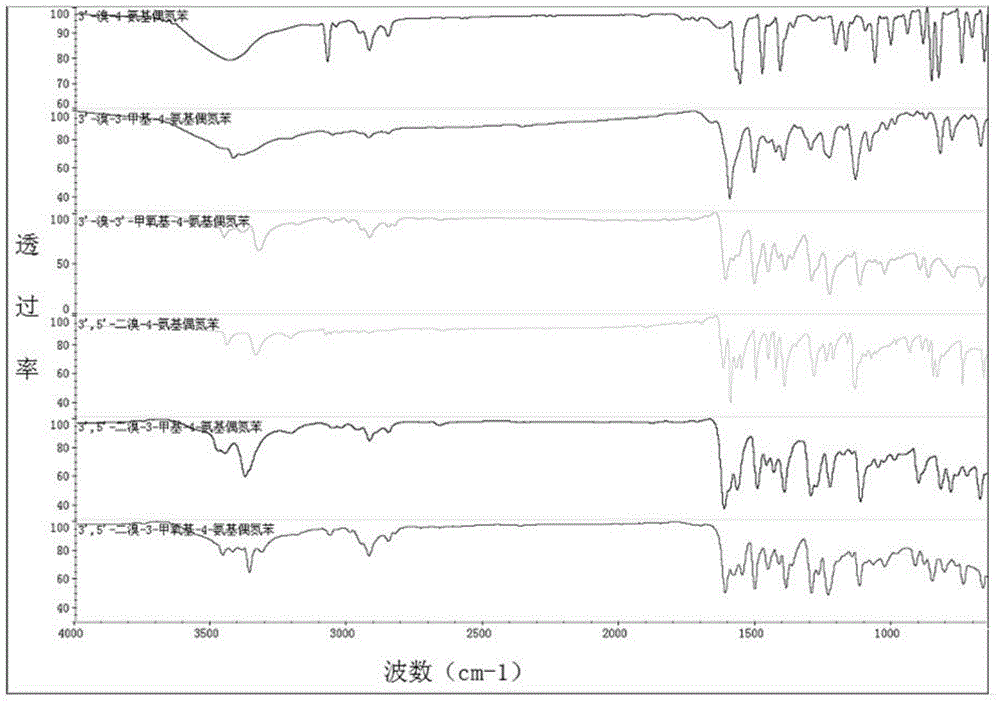
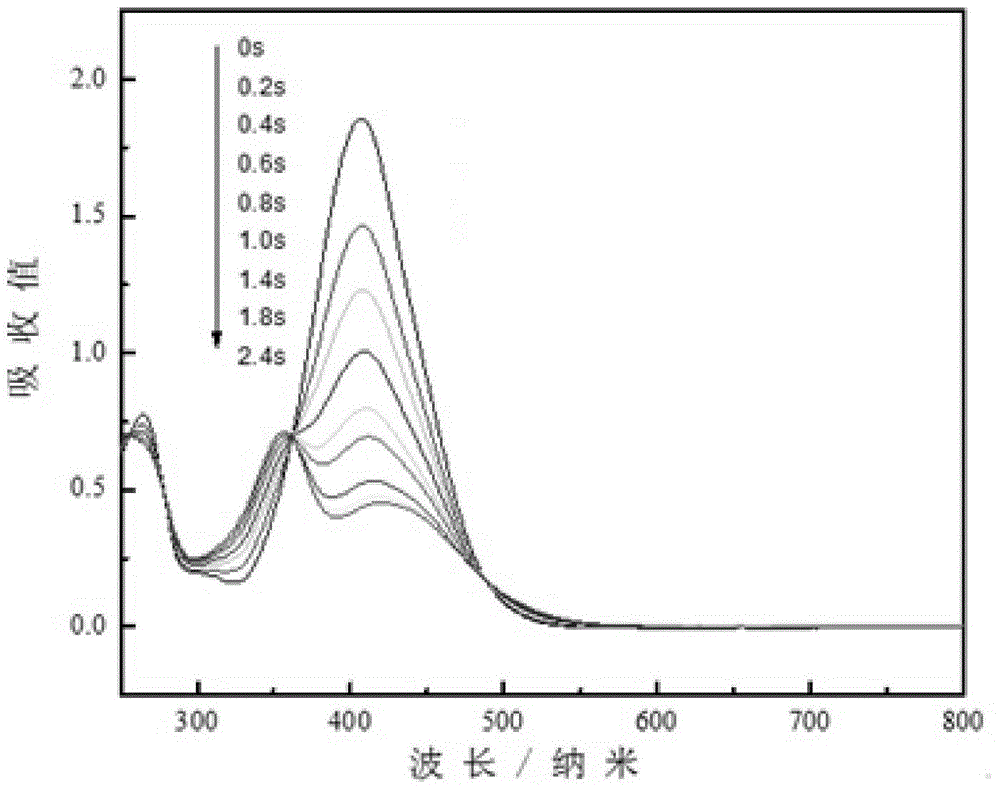
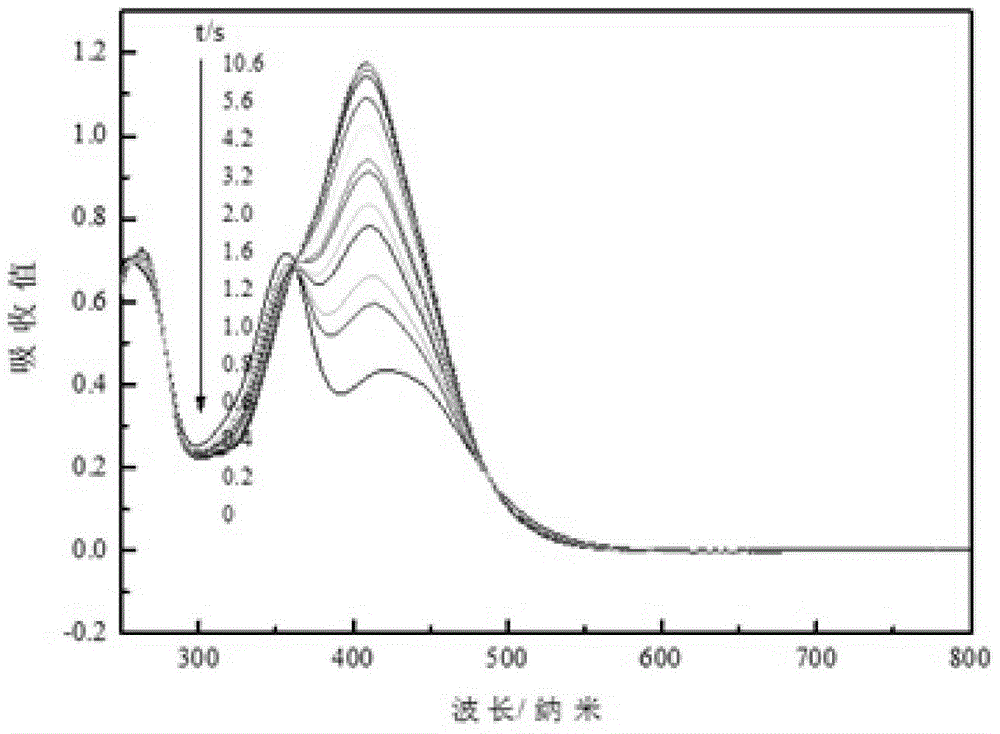
D-pi-A-type aminoazobenzene dye and preparation method therefor
A technology of aminoazobenzene and azobenzene, which is applied in the direction of azo dyes, monoazo dyes, organic dyes, etc., and can solve the problem of less blue light azo dyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The present embodiment is the preparation method of 3?-bromo-4-aminoazobenzene, comprising the following steps:
[0043] (1) Preparation of diazonium salt: Add 3.4408 g of 3-bromoaniline and 8 mL of 20% hydrochloric acid into a 100 mL beaker, slowly add 1.5201 g of sodium nitrite aqueous solution dropwise at 0-5 °C, add urea after 2 h to eliminate excess nitrite Nitric acid and solid sodium acetate were used to adjust the pH value to 5, and the obtained clarified solution was placed in ice water for later use.
[0044] (2) Preparation of sodium anilinomethanesulfonate: add 10 mL of water and 2.3505 g of sodium bisulfite to a 50 mL round bottom flask, add 0.7251 g of paraformaldehyde after dissolving the sodium bisulfite, react at 60°C for 35 min, then drop Add 1.8611 g of aniline that had been distilled, and stop heating after reacting for 2 h to obtain a mixed solution of sodium anilino methanesulfonate.
[0045] (3) Preparation of azobenzene compound: After...
Embodiment 2
[0048] The present embodiment is the preparation method of 3?-bromo-3-methyl-4-aminoazobenzene, comprising the following steps:
[0049] (1) Preparation of diazonium salt: Add 3.4414 g of 3-bromoaniline and 8 mL of 20% hydrochloric acid into a 100 mL beaker, slowly add 1.5216 g of sodium nitrite aqueous solution dropwise at 0-5 °C, add urea after 2 h to eliminate excess nitrite Nitric acid and solid sodium acetate were used to adjust the pH value to 5, and the obtained clarified solution was placed in ice water for later use.
[0050] (2) Preparation of sodium o-toluanilino methanesulfonate: add 10 mL of water and 2.3517 g of sodium bisulfite to a 50 mL round bottom flask, add 0.7264 g of paraformaldehyde after dissolving sodium bisulfite, and react at 60°C for 35 After 2 min, 2.1406 g of o-methylaniline was added dropwise, and the heating was stopped after 2 h of reaction to obtain a mixed solution of sodium o-toluinomethanesulfonate.
[0051] (3) Preparation of azobenzene c...
Embodiment 3
[0054] The present embodiment is the preparation method of 3?-bromo-3-methoxy-4-aminoazobenzene, comprising the following steps:
[0055] (1) Preparation of diazonium salt: Add 3.4451 g of 3-bromoaniline and 8 mL of 20% hydrochloric acid into a 100 mL beaker, slowly add 1.5211 g of sodium nitrite aqueous solution dropwise at 0-5 °C, add urea after 2 h to eliminate excess nitrite Nitric acid and solid sodium acetate were used to adjust the pH value to 5, and the obtained clarified solution was placed in ice water for later use.
[0056] (2) Preparation of sodium o-methoxyanilino methanesulfonate: add 10 mL water and 2.3528 g sodium bisulfite to a 50 mL round bottom flask, add 0.7242 g paraformaldehyde after dissolving sodium bisulfite, and react at 60 °C After 35 min, 2.4608 g of o-methoxyaniline was added dropwise, and the heating was stopped after 2 h of reaction to obtain a mixed solution of sodium o-methoxyanilino methanesulfonate.
[0057] (3) Preparation of azobenzene co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com