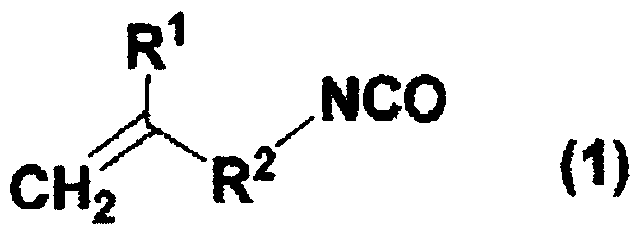
Polymers containing blocked isocyanate groups, compositions containing the same and uses thereof
An isocyanate-based, polymer technology, applied in the direction of polyurea/polyurethane coating, photo-engraving process of pattern surface, photosensitive material used in opto-mechanical equipment, etc. High utility value, excellent sensitivity and developability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] 162.2 g of propylene glycol monomethyl ether acetate was placed in a flask equipped with a stirring device, a dropping funnel, a condenser, a thermometer, and a gas introduction tube, and the temperature was raised to 120° C. while stirring while replacing nitrogen.
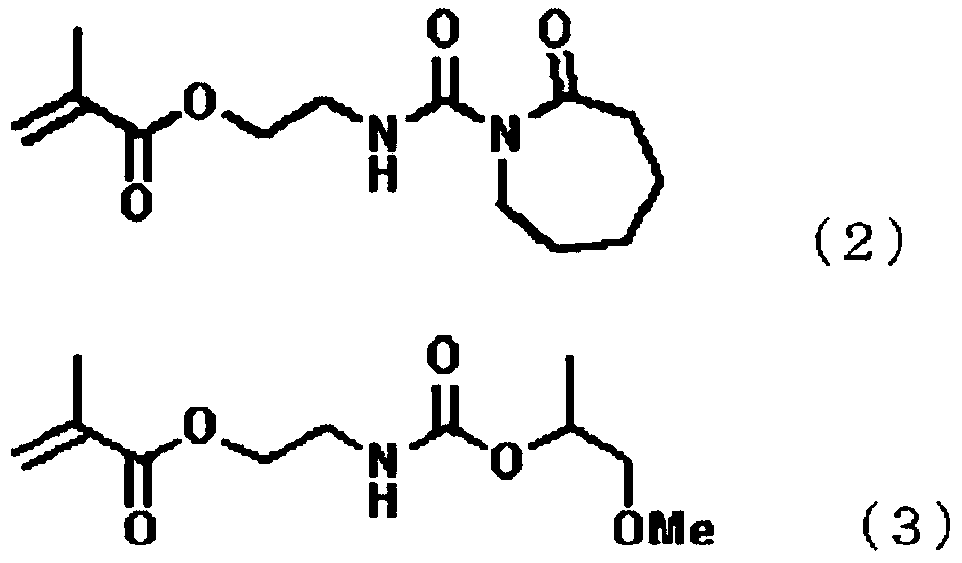
[0131] Next, methacryloyloxyethyl isocyanate represented by 4.7g (0.05 mol) of norbornene, 53.0g (0.45 mol) of vinyltoluene, 32.4g (0.45 mol) of acrylic acid and the above-mentioned formula (3) and 10.3 g of tert-butylperoxy-2-ethylhexanoate (polymerization initiator, Japanese Oil Co., Ltd. (Parbull O), and the obtained solution was dropped into the above-mentioned flask from the dropping funnel over 2 hours. After completion|finish of dripping, it stirred at 120 degreeC for 2 hours, and copolymerization reaction was performed, and the addition copolymer (precursor of curable polymer) was produced|generated. Then, the inside of the flask was replaced with air, 22.5 g (0.16 mol) of glycidyl methacrylate, 0...
Embodiment 2
[0134] 232.8 g of propylene glycol monomethyl ether acetate was added to a flask equipped with a stirring device, a dropping funnel, a condenser, a thermometer, and a gas introduction tube, and the temperature was raised to 120° C. while stirring while replacing nitrogen. Next, in 6.09g (0.06 mole) of norbornene, 68.33g (0.58 mole) of vinyltoluene, 82.0g (0.58 mole) of glycidyl methacrylate and the methacryloyl oxide represented by the above formula (3) 13.3 g of tert-butyl peroxy-2-ethylhexanoate ( Polymerization initiator, manufactured by NOF Corporation, Perbucell (2), and the obtained solution was dropped into the above-mentioned flask from the dropping funnel over 2 hours. After completion of the dropwise addition, the mixture was further stirred at 120° C. for 2 hours to perform a copolymerization reaction to produce an addition copolymer. Then, the inside of the flask was replaced with air, and 42.0 g (0.58 mol) of acrylic acid, 0.5 g of triphenylphosphine (catalyst) a...
Embodiment 3
[0137] Use 13.4 g (0.05 mol) of the reaction product (dissociation temperature 160°C) of 2-isocyanatoethyl methacrylate represented by the above formula (2) and ε-caprolactam instead of the methyl group represented by the above formula (3) A polymerizable polymer (C) was obtained in the same manner as in Example 1 except that the reaction product of acryloyloxyethyl isocyanate and propylene glycol monomethyl ether was 12.25 g (0.05 mol). The acid value of the polymer was 130.2 mgKOH / g, the unsaturated group equivalent was 859 g / mol, the blocked isocyanate group equivalent was 2700 g / mol, and the weight average molecular weight was 15900.
[0138] Next, 106.53 g of propylene glycol monomethyl ether was added to the reaction solution to prepare a polymer solution in which the concentration of the polymer portion of the polymerizable polymer (C) was 34%. Let this be sample 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com