Application of 2H-1-chromene-2-ketone in medicine preparation
A benzopyran and drug technology, which can be used in drug combinations, antipyretics, antitumor drugs, etc., can solve problems such as inconsistent TLR4 expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
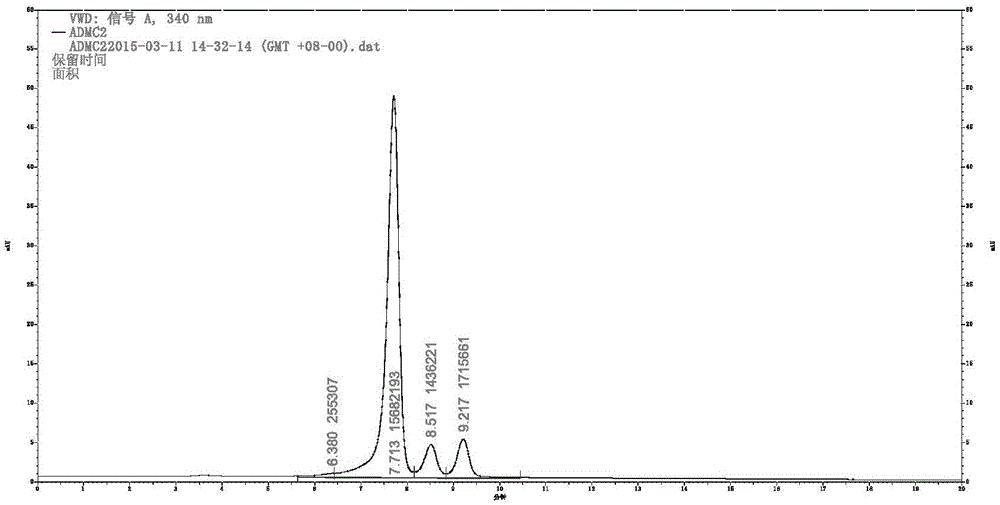
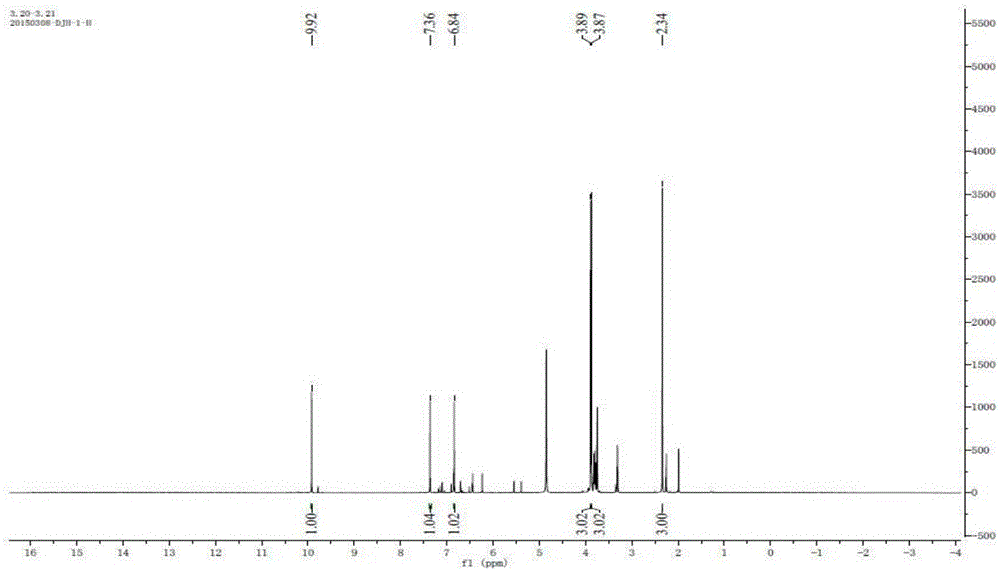
[0024] Synthesis and Structure Identification of 2H-1-Benzopyran-2-one
[0025] 1 material
[0026] 1.1 Reagents
[0027] 2-Hydroxy-4,5-dimethylbenzaldehyde was purchased from Shaoyuan Technology (Shanghai) Co., Ltd.; ethyl acetoacetate, methanol (high performance liquid chromatography), piperidine, and deuterated methanol were purchased from Sinopharm Group Co., Ltd. ; Purified water was purchased from Wahaha Group Co., Ltd.
[0028] 1.2 Instruments
[0029] Bruker AM-400 (400MHz) nuclear magnetic resonance instrument was purchased from Bruker AG of Switzerland; high performance liquid chromatography (G4288C, 1220LC system VL) was purchased from Agilent Technologies Co., Ltd. of the United States
[0030] 2 methods
[0031] 2.1 Synthesis method
[0032] Mix 2-hydroxy-4,5-dimethoxybenzaldehyde (2g) with excess ethyl acetoacetate, add 0.5ml of cooled piperidine, react at a temperature below room temperature, and precipitate yellow in ethyl acetate after 24h Matter is a pr...
Embodiment 2
[0043] Effects of 2H-1-benzopyran-2-one on the proliferation and apoptosis of mouse lymphocytes and the surface antigens of B lymphocytes and primary DC
[0044] 1 material
[0045] 1.1 Animals
[0046] BALB / c mice, C57BL / 6 mice, purchased from the Animal Center of Tongji Medical College, Huazhong University of Science and Technology, 6-8 weeks old, male.
[0047] 1.2 Reagents
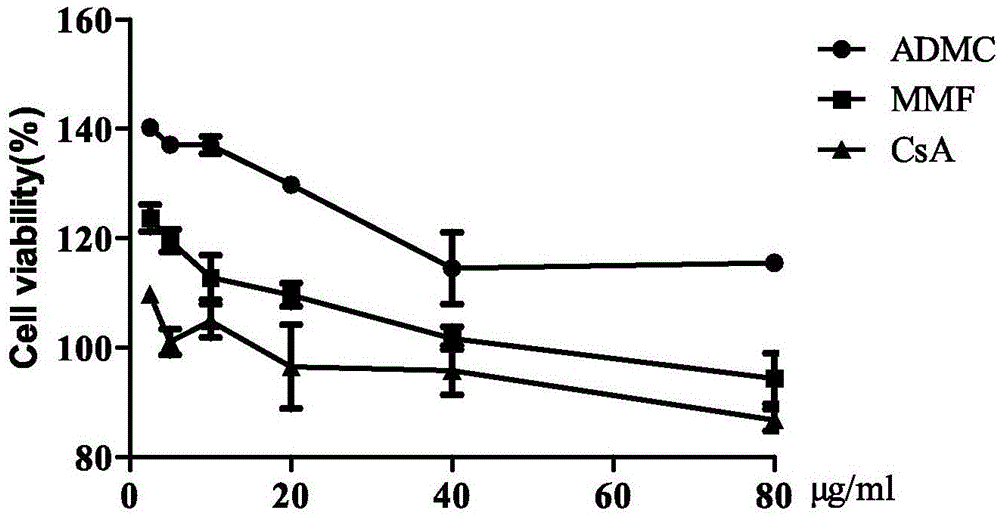
[0048] Concanavalin A (Con A), lipopolysaccharide (LPS), cyclosporine A (CsA), mycophenolate mofetil (MMF) and thiazolyl blue (MTT) were purchased from Sigma Company of the United States; RPMI-1640 medium was purchased from the United States HyClone Company, fetal bovine serum (FBS), double antibody, purchased from Gibco Company of the United States; PI kit, purchased from Hubei Biosimilar Company; GM-CSF, purchased from SAB Company of the United States; APC-labeled Anti-CD11c flow antibody , FITC-labeled Anti-CD86 flow-type antibody, PE-labeled Anti-MHC-II flow-type antibody, purchased from eBiosci...
Embodiment 3
[0065] Effects of 2H-1-benzopyran-2-one on LPS-induced secretion of NO and TNF-α from RAW264.7 cells
[0066] 1 material
[0067] 1.1 Cell lines
[0068] The mouse mononuclear macrophage RAW264.7 cell line was purchased from the cell experiment platform of Tongji Medical College, Huazhong University of Science and Technology.
[0069] 1.2 Reagents
[0070] Lipopolysaccharide (LPS) was purchased from Sigma Company of the United States; RPMI-1640 medium was purchased from HyClone Company of the United States; fetal bovine serum (FBS) and double antibodies were purchased from Gibco Company of the United States; nitric oxide detection kit was purchased from China Biyuntian Biotechnology Research Institute; enzyme-linked immunoassay kit for quantitative analysis of mouse TNF-α was purchased from Shanghai Qiaoyi Biotechnology Co., Ltd. Antibody to TLR4 protein was purchased from Santa Cruz, USA; DMSO (dimethyl sulfoxide) was purchased from Beijing Dingguo Changsheng Biotechnology...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ic50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com