Pristane synthesizing method
A synthetic method, pristane technology, applied in the field of biological reagents, to achieve the effect of low cost of raw materials, cheap raw materials, high cost of separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
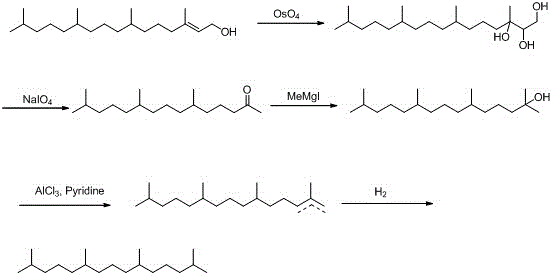
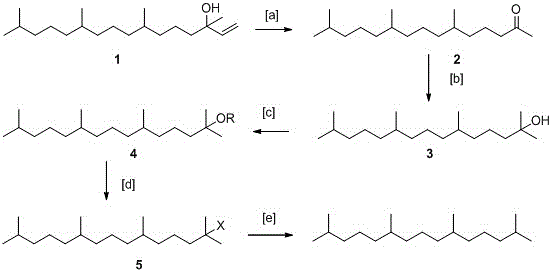
[0034] (1) Oxidation reaction, synthesis of phyton 2:
[0035] First, add 250g of isophytol 1, 2L of acetone, and 50ml of acetic acid into a 5L reaction flask in sequence. Slowly add 501g KMnO in batches 4 . With the prolongation of the reaction time, there will be exothermic phenomenon gradually. Cool with a little water circulation until the addition is complete, continue the reaction for 5h, then heat at 60°C and continue the reaction for 5h, after the reaction is complete, filter, extract with ethyl acetate, and dry over anhydrous sodium sulfate . 212g of phyton 2 was obtained, yield: 93%.
[0036] The NMR spectrum of Phytoketone 2 is:
[0037] 1 HNMR (500MHz, CDCl 3 ): 2.42-2.38 (2H, t, CH2), 2.13 (3H, s, CH3), 1.54-1.51 (3H, m, CH), 1.26-1.25 (2H, m, CH2), 1.15-1.07 (16H, m, CH2), 0.88-0.84 (12H, m, CH3).
[0038] (2) Methyl Grignard reaction, synthesis of compound 3:
[0039] Put 200g of phyton 2 into a 3L dry reaction flask, vacuumize, fill with N2, add 1.5L o...
Embodiment 2
[0051] (1) Oxidation reaction, synthesis of phyton 2:
[0052] First, add 300g of isophytol 1, 2.5L of acetone, and 100ml of acetic acid into a 5L reaction flask in sequence. Slowly add 454g H in batches 2 Cr 2 o 7 . As the reaction time prolongs, exothermic phenomenon gradually occurs, and the reaction is continued for 6 hours until the feeding is completed, and the reaction is continued for 8 hours. The rest is the same as in Example 1, and the yield is 93%.
[0053] (2) Methyl Grignard reaction, synthesis of compound 3:
[0054] Put 200g of phyton 2 into a 3L dry reaction bottle, vacuumize and fill with N 2 , add 1.5L of anhydrous tetrahydrofuran, cool to -10°C, slowly add 298ml of 3mol / L methyl Grignard reagent MeMgCl dropwise, after the dropwise addition, naturally warm up to room temperature, continue stirring for 1h, and the rest are the same as in Example 1 , yield: 93%, b.p.: 323°C.
[0055] (3), sulfonylation reaction, synthesis of compound 4:
[0056] Add 18...
Embodiment 3
[0062] (1) Oxidation reaction, synthesis of phyton 2:
[0063] First, add 200g of isophytol 1, 1.5L of acetone, and 40ml of acetic acid into a 5L reaction flask in sequence. Slowly add 426gNaIO 4 . With the prolongation of the reaction time, there is a gradual exothermic phenomenon, and the water is circulated and cooled slightly until the addition is completed, and the reaction is continued for 4 hours, and then heated to 80° C. to continue the reaction for 4 hours. The rest is the same as in Example 1, and the yield is 95%.
[0064] (2) Methyl Grignard reaction, synthesis of compound 3:
[0065] Put 180g of phyton 2 into a 3L dry reaction bottle, vacuumize and fill with N 2 , add 1.5L of anhydrous tetrahydrofuran, cool to -10°C, slowly add 298ml of 3mol / L methyl Grignard reagent MeMgI dropwise, after the dropwise addition, naturally warm up to room temperature, continue stirring for 3h, and the rest are the same as in Example 1 , yield: 96%, b.p.: 322°C.
[0066] (3), s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com